Question
Question: What do you mean by close packing?...
What do you mean by close packing?
Solution
The close packing structures are present in solids because the spaces between the solids are very less due to which the atoms are very close to each other. In close packing, there are many types like close packing in one-dimension, close packing in two-dimension, etc.
Complete step-by-step answer: Close packing is a type of arrangement of the atoms or molecules in the compound. This arrangement is present in solids, i.e., crystals. The close packing structures are present in solids because the spaces between the solids are very less due to which the atoms are very close to each other. To understand the close packing in the solids, the atoms are molecules that are considered spherical. The atoms and molecules are arranged in a cubical crystal in which they arrange themselves to occupy maximum space which makes the solids denser, stronger, and harder.
There are many types of close packing, like close-packing in one-dimension, close packing in two-dimension, etc.
The close packing in one-dimension is when the atoms or molecules are in a straight line, i.e., in a single row. This is shown below:
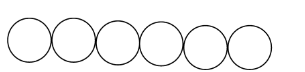
The close packing in two-dimension is when one layer of the molecules is over another layer. This is done in two ways when the molecule of one layer is exact over the other layer and it is called square close packing.
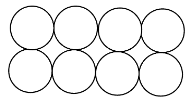
The other way is when the molecules of one layer are over the space between the molecules of the second layer and it is called hexagonal close packing. As shown below:
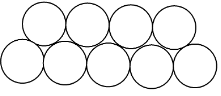
Note: As we can see from the diagrams that the space between the molecules in square close packing is more than the space between the hexagonal close packing.
