Question
Question: What covalent compound is \( {P_4}{S_5} \) ?...
What covalent compound is P4S5 ?
Solution
Hint : The name of the compound with the formula P4S5 is tetraphosphorus pentasulfide. Covalent compounds are also known as molecular compounds. They are made up of elements which are connected to each other by covalent bonds.
Complete Step By Step Answer:
The elements phosphorus and sulphur form the compound P4S5 . Tetraphosphorus pentasulfide is formed when a solution of tetraphosphorus trisulfide also called phosphorus sesquisulfide, sulphur and some amount of iodine in dry carbon disulphide are exposed to diffuse daylight for a period of three days. This chemical reaction has to be carried in a closed vessel in an atmosphere of carbon dioxide to prevent the oxidation of tetraphosphorus trisulfide. The substance can be purified by the process of recrystallization from carbon disulfide. X-ray photographs were made from the crystals having a radius of approximately 0.05mm , perpendicular to the axis about which the photographs were taken. The crystals were mounted in capillaries of borosilicate glass. The compound tetraphosphorus pentasulfide is a covalent compound as the molecule is formed by covalent bonds, in which the atoms share one pair or more than one pair of valence electrons. The structure of the compound tetraphosphorus pentasulfide is given below:
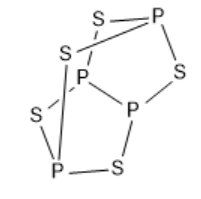
Note :
In the structure of the compound tetraphosphorus pentasulfide, there are eleven covalent bonds. There are five atoms of sulphur and four atoms of the element phosphorus. The molecular weight of tetraphosphorus pentasulfide is 284.195 g mol - 1 . The complexity of tetraphosphorus pentasulfide is 120 . The monoisotopic mass of the compound tetraphosphorus pentasulfide is 283.755 g mol - 1 .
