Question
Question: What conclusions would you draw from the following graphs : 
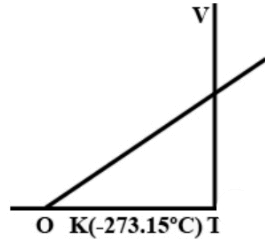
a.) As the temperature is reduced, the volume as well as pressure increase
b.) As the temperature is reduced, the volume becomes zero and the pressure reaches infinity
c.) As the temperature is reduced, the volume as well as pressure decreased
d.) A point is reached where theoretically, the volume as well as the pressure become zero.
Solution
We see that in the graph, the pressure and volume is linearly decreasing with decrease in temperature. If the line touches the temperature line, at that time, the value of property is zero. So, at 0 K temperature, the value of pressure and volume is zero.
Complete answer:
We have been given pressure-temperature and volume-temperature graphs.
If we observe both the graphs we see that moving right to left, the temperature is decreasing. And with decrease in temperature the amount of pressure and volume is also decreasing linearly with the temperature.
Further, we see that a point comes when the pressure becomes zero and even in the volume-temperature graph, a point comes where the volume is zero.
This point in both graphs is at 0 K temperature. This is called absolute zero.
Practically, it is not possible that the volume of the solution or gas is zero. Thus, it is a theoretical consideration. So, at the absolute zero, volume, pressure and kinetic energy etc. is zero.
So, the correct answer is option c.) and d.).
Note:
It must be noted that 0 K is called absolute zero temperature. It is the lowest limit of the thermodynamic temperature scale. At this state, the value of enthalpy and entropy of a cooled ideal gas reach minimum value.
