Question
Question: What color change will you observe in the test tubes and why? If in an experiment, \(N{O_2}\) is pre...
What color change will you observe in the test tubes and why? If in an experiment, NO2 is prepared and kept in 3 text tubes X,Y and Z. NO2 gas is brown in color which dimerizes into N2O4 which is colorless. X is kept at room temperature, Y in ice and Z in hot water. If 2NO2(g)⇌N2O4(g),ΔH=−57.2kJmol−1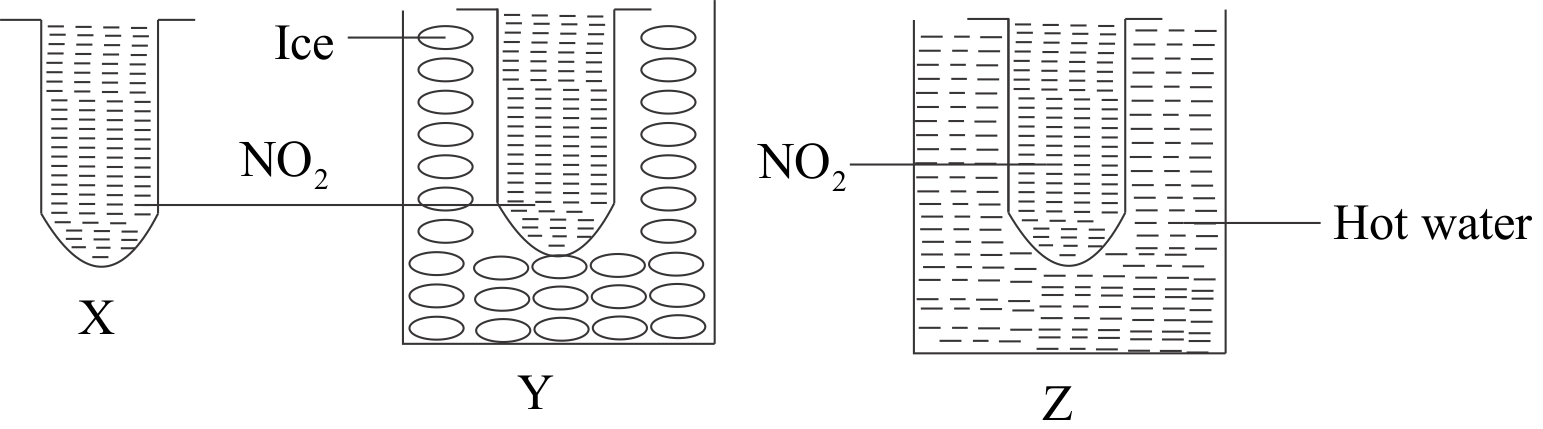
A.In test tube X, brown color intensifies since the backward reaction is favoured at low temperature.
B.In test tube Y, brown color intensifies since backward reaction takes place at room temperature.
C.In test tube Z, brown color intensifies since high temperature favours the backward reaction.
D.Brown color in the test tubes X, Y and Z remains the same since there is no effect of change in the temperature on the reaction.
Solution
Le Chatelier's Principle helps to predict what effect a change in temperature, concentration or pressure will have on the position of the equilibrium in a chemical reaction.
Complete answer:
Since this is an exothermic process, heat is released, and at low temperatures forward reaction is favoured.
In test tubes, X and Y forward reaction is favoured and N2O4 is formed which is colorless. In the test tube, brown color intensifies, as it is kept in hot water since high temperature favours the backward reaction.
Hence, the correct answer is (C).
Note:
-If the temperature of a reaction mixture is changed, the equilibrium will shift to minimize that change.
-If the temperature is increased, the equilibrium will shift to favour the reaction which will reduce the temperature. The endothermic reaction is favoured.
-If the temperature is decreased, the equilibrium will shift to favour the reaction which will increase the temperature. The exothermic reaction is favoured.
