Question
Question: What characteristics do you expect from an electron-deficient hydride with respect to its structure ...
What characteristics do you expect from an electron-deficient hydride with respect to its structure and chemical reactions?
Solution
Hint : The electron deficient nature mentioned in the question is about the compounds that elements in group thirteen form. To be exact, the elements being pointed out are Aluminium and Boron.
Complete step by step solution :
The octet rule only applies to the elements which have atomic numbers below twenty. In other words, the elements belonging to d and f blocks are not included. The Octet rule holds true only for the elements in second and third periods. This rule states that a chemical reaction between elements occurs so as to satisfy their octets, i.e. they have to maintain an exact eight number of electrons in their valence shells in order to be stable molecules. This theory has many exceptions and one of them is the fact that there are certain molecules which contain electron deficient atoms which have less than valence eight electrons in their valence shells.
A common example is the compounds of the elements belonging to group thirteen, specifically aluminium and boron. The Lewis structure of aluminium trichloride (AlCl3) is as follows:
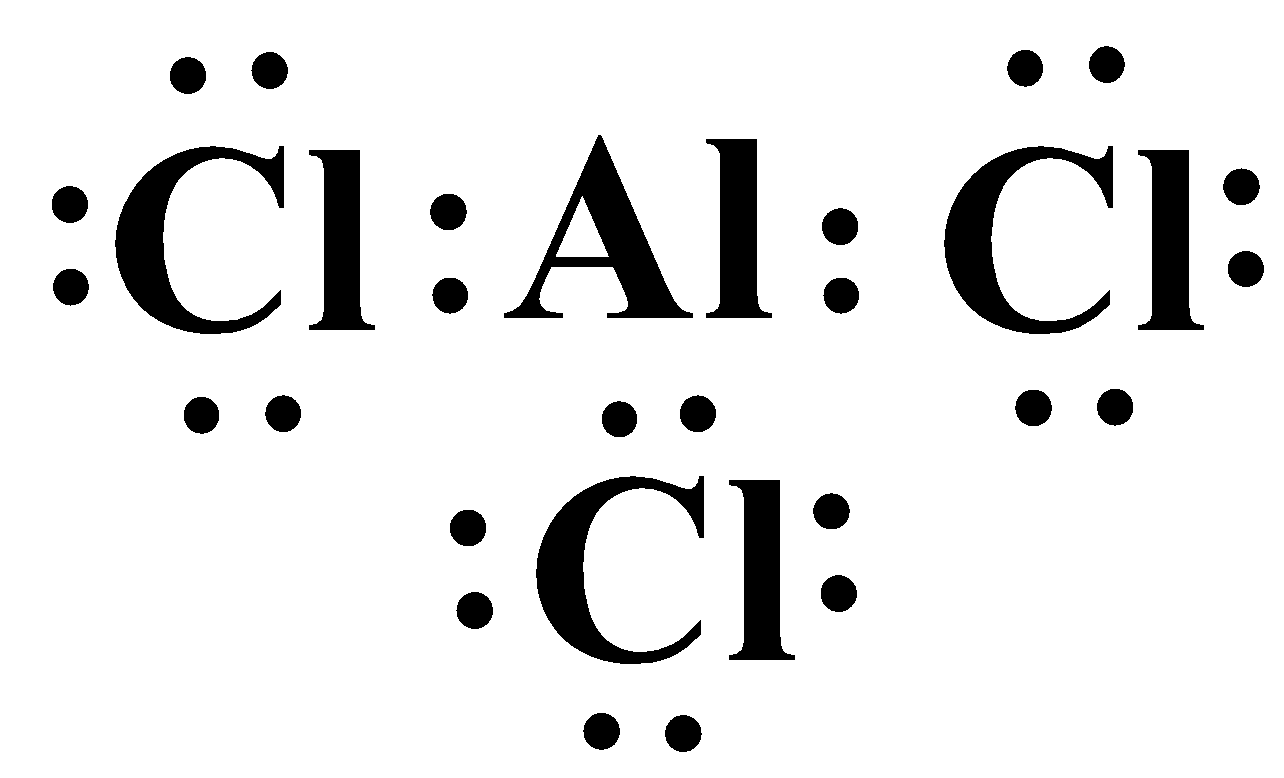
As you can see, the valence shell of the metal only contains six electrons which completely violates the octet rule and yet this molecule exists and is stable. It is also seen that the compound aluminium tetrachloride (AlCl4−) is also a stable compound. Here the metal has a complete octet, because of the negative charge of chloride ion.
The same phenomenon happens with aluminium hydrides. As mentioned above, Boron also faces similar issues. It is also an electron deficient molecule as it lacks two electrons to complete its octet.
Thus the hydrides of both aluminium and boron are electron deficient in nature. Implying they are very good Lewis acids. A Lewis acid can be defined as an electron acceptor. Boron hydride accepts another hydride ion to become BH4−. In this molecule Boron has a complete octet with eight electrons. The Lewis structure is as shown below:
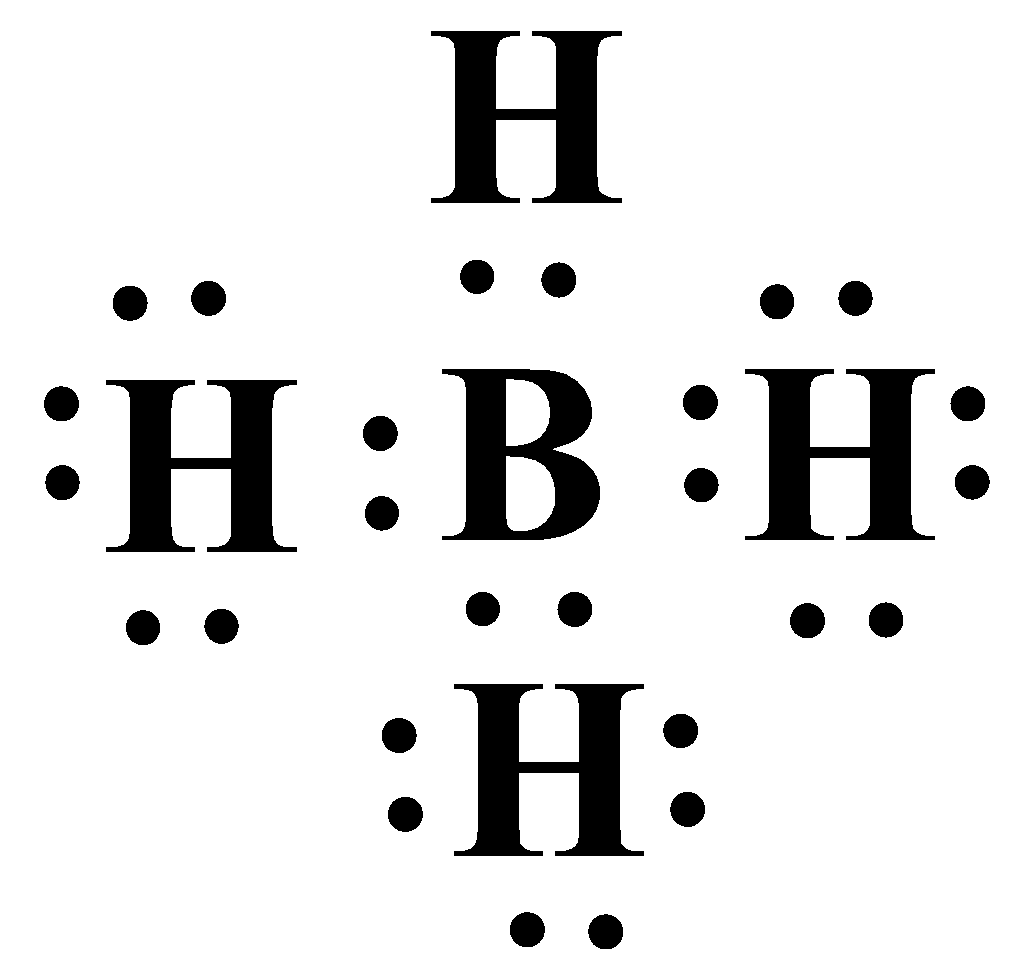
An electron deficient hydride will therefore behave as a potent Lewis acid.
Note : Not all the elements of group thirteen behave in this way. It is because the octet rule does not apply to them. Elements starting from the fourth period have electrons filling the d orbitals. Elements present here show multiple valencies because of their many stable oxidation states.
Although the metal aluminium has a vacant d orbital, no reaction or phenomenon occurring with this element uses them.
