Question
Question: What \({C_9}{H_{12}}\) hydrocarbon would give a single \({C_9}{H_{11}}S{O_3}H\) product on sulphonat...
What C9H12 hydrocarbon would give a single C9H11SO3H product on sulphonation?
A) 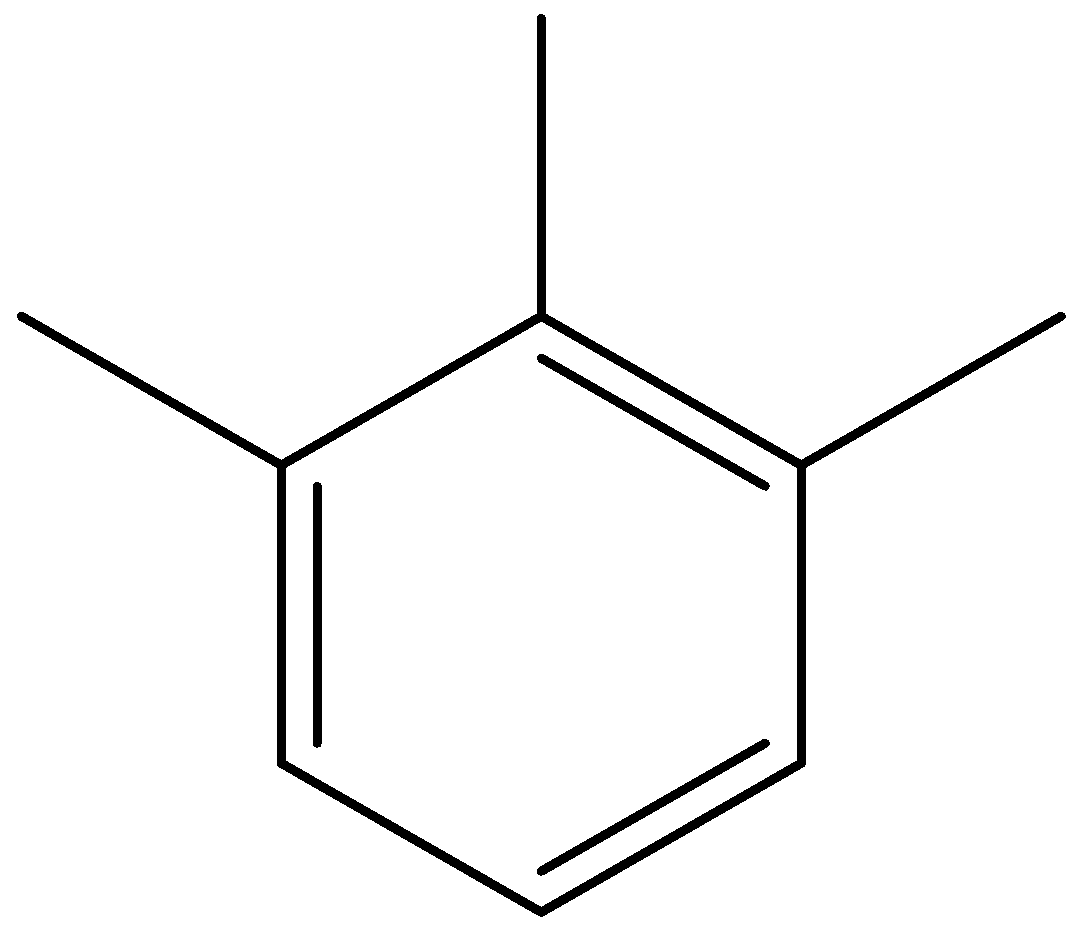
B) 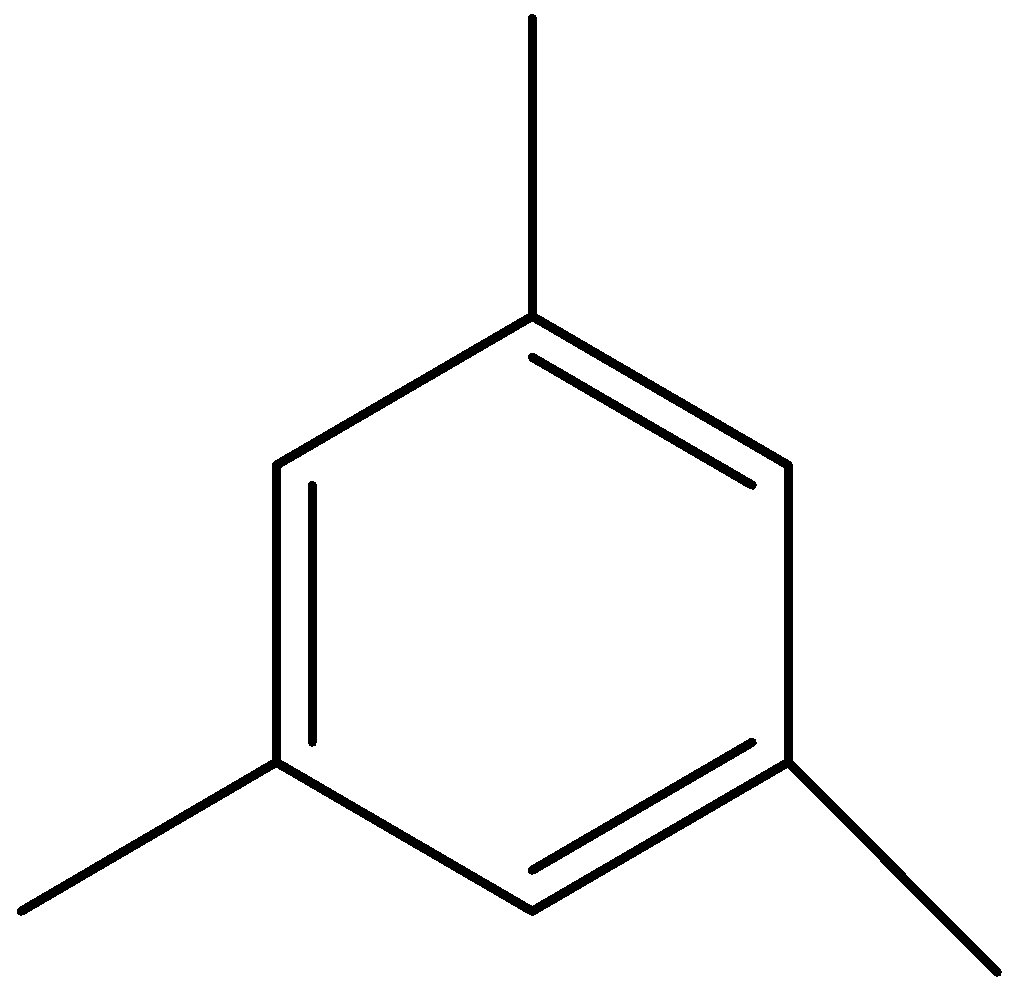
C) 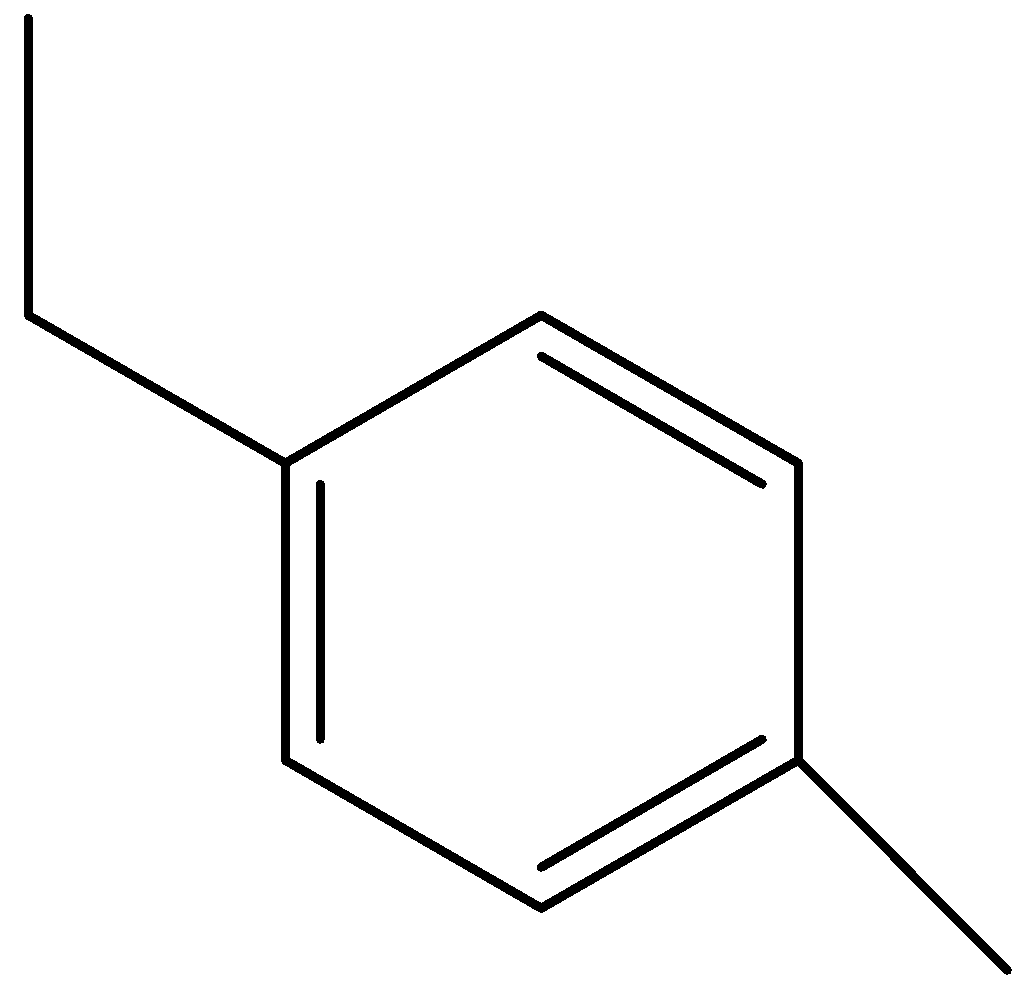
D) 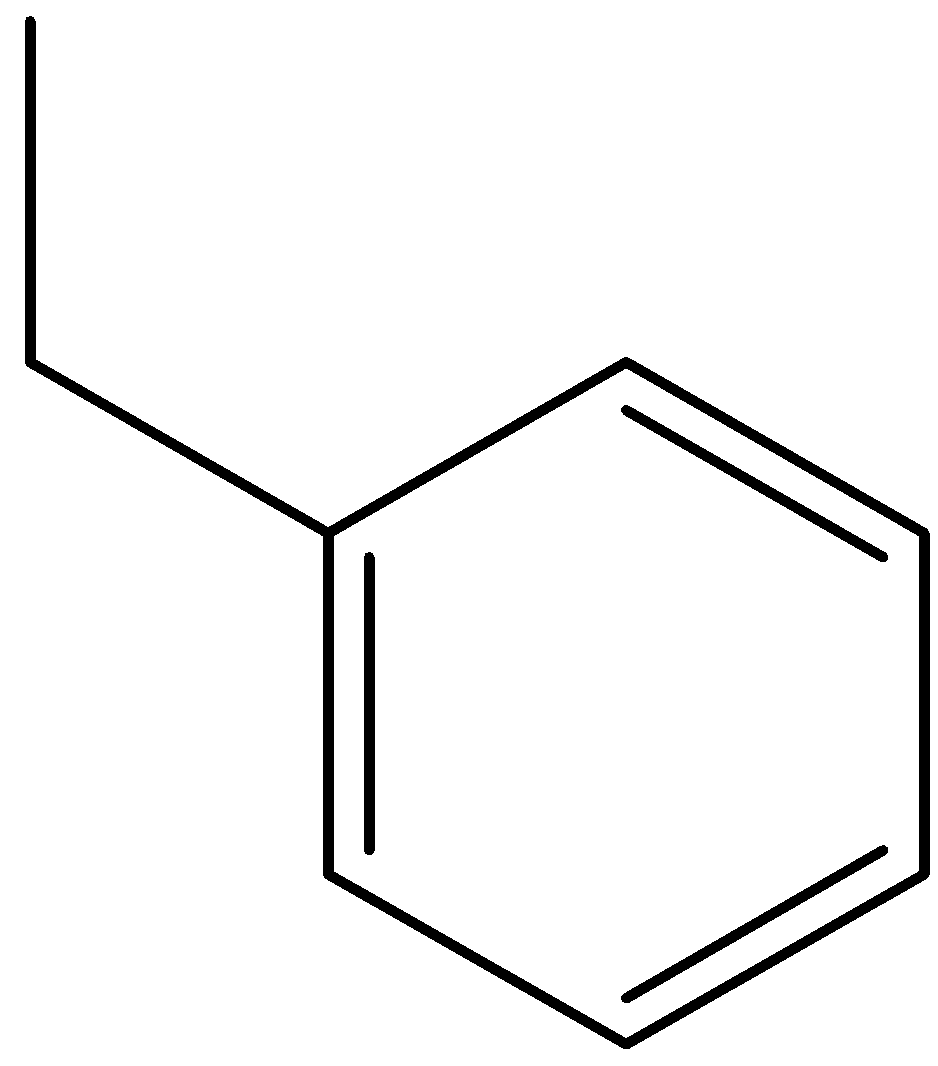
Solution
We know that whenever an atom of hydrogen present on an arene is replaced by sulfonic acid functional group in an electrophilic aromatic substitution is known as aromatic sulfonation reaction.
Complete step by step answer:
We know that all the aromatic compounds are derivatives of benzene. A benzene ring has six carbon atoms. The remaining carbons are left as an attached group such as methyl, ethyl to the benzene ring.
When the three carbon leaves are seen as three methyl groups attached to a benzene ring, then the compound is mesitylene.
The hydrocarbon with molecular formula C9H12 which would give a single product C9H11SO3H on sulphonation reaction is mesitylene.
We can draw the structure of mesitylene as,
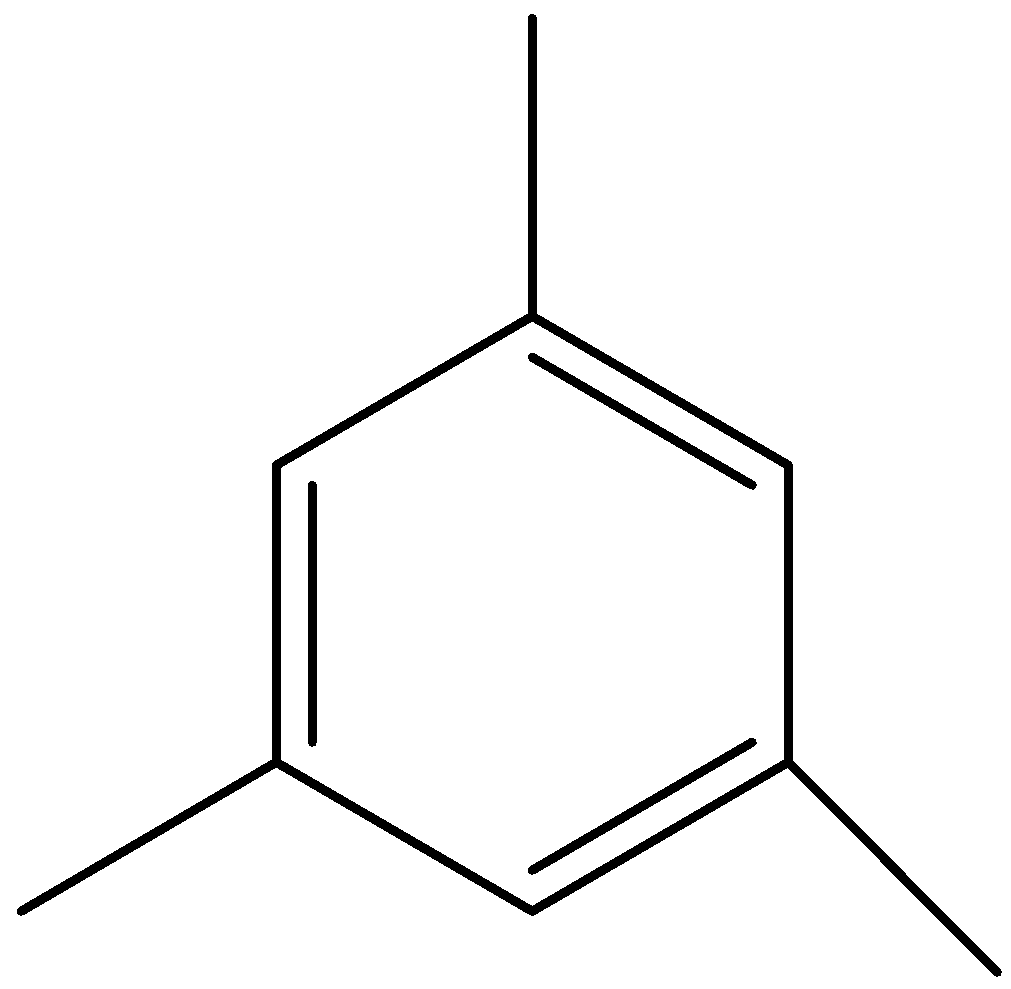
We can see that in mesitylene, all positions are equal, so there would be a single product formed on sulphonation.
Therefore, the option (B) is correct.
Option (A) is 1,2,6-trimethylbenzene.
Option (C) is 1-ethyl-4-methylbenzene.
Option (D) is ethylbenzene.
In other compounds when electrophilic aromatic substitution takes place, two products are formed. One of the products formed will be a major product and the other product formed would be minor. These products are ortho compounds and para compounds.
Therefore, the option (A), (C) and (D) are incorrect.
Therefore, the option (B) is correct.
Note:
We can use aryl sulfonic acid in drugs, detergents, and dyes. We can use chlorosulfonic acid as an effective agent in sulfonation reactions. We can add dehydrating agents like thionyl chloride to drive the equilibrium. When we sulphonate polystyrene, we can obtain sodium polystyrene sulfonate. A large group of sulfa drugs can be obtained by sulfonation of anilines.
