Question
Question: What atomic or hybrid orbitals make up the sigma bond between \[I\] and \[Cl\] in iodine pentachlori...
What atomic or hybrid orbitals make up the sigma bond between I and Cl in iodine pentachloride, ICl5?
Solution
The intermixing of two or more pure atomic orbitals of an atom with almost the same energy to give the same number of identical and degenerate new types of orbitals is known as hybridization. The new orbitals formed are also known as hybrid orbitals.
Formula to find the hybridization of a molecule is given by
Hybridisation=Number of σ bonds+ Number of lone pairs
Complete answer:
If the sum is 2 → hybridization − sp
If the sum is 3 → hybridization − sp2
If the sum is 4 → hybridization − sp3
If the sum is 5 → hybridization− sp3d
If the sum is 6 → hybridization− sp3d2
COMPLETE STEP BY STEP ANSWER-
Lewis dot structure of ICl5:
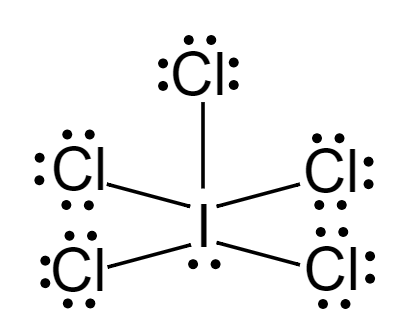
Considering central metal atom, I
Number{\text{ }}of{\text{ }}lone{\text{ }}pairs = 1 \\\
Number{\text{ }}of{\text{ }}bond{\text{ }}pairs = 5 \\\
Hybridisation=1+5=6
Therefore, I−Cl bond is sp3d2 hybridized.
About ICl5,
Hybridisation-sp3d2
Geometry- Octahedral
Shape- Square pyramidal
Note:
You can expect bond angles of 90∘ and 180∘.
Also note that when forming hybrid orbitals, you use n atomic orbitals to make n hybrid orbitals, where n is the total amount of orbitals. Here, in case of ICl5, n=6
