Question
Question: What are Y and Z in the following reaction sequence? 
A. Y-ethyne, Z-acetic acid
B. Y-ethyne, Z-ethanal
C. Y-ethylene, Z-ethanal
D. Y-ethane, Z-ethanol
Solution
NaNH2dissociates into Na+ and NH2−. The NH2− reacts with the alkyl halide giving the product Y. The product Y will help us to find Z accordingly.
Complete step-by-step answer:
The given reaction is,
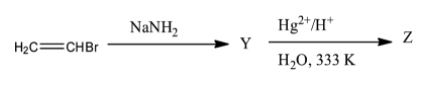
To find Y and Z we will consider the following stepwise reactions.
Step 1:
Sodium amide, NaNH2 dissociates into a sodium ion, Na+and NH2−.

Step 2:
The NH2− ion then reacts with the ethyl bromide to generate the product Y. Product Y is an ethyne.
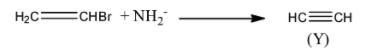
Step 3:
Now, this ethyne will react with Hg2+/H+ and water at 300∘C generating the product Z.

The product Z is ethanal.
Alkyl halide is marked by the presence of a halide group with an alkyl group, that is presence of bromide with the ethene molecule gives the name to the compound C2H3Br, ethyl bromide. Ethyne is marked by the presence of triple bonds between the two carbon atoms. Ethanol is marked by the presence of an aldehyde group. The conversion of the ethyne to the ethanal requires a temperature of 300∘C and in this reaction, water is used as the solvent.
So, out of the given options, B is the correct option, that is, Y-ethyne, Z-ethanal.
Note: Students may find it difficult to predict multiple products in a reaction. So, we should first start with the product that comes first. The finding of the first product will have to find the second product.
