Question
Question: What are X and Y 
1.
2.
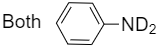
3.
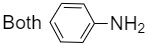
4.
Solution
Hint : Benzoic acid, a simple aromatic carboxylic acid with molecular formula C6H5COOHis a white solid having monoclinic crystal structure.
Acid amides are quite reactive towards nucleophilic substitution. This is because of the positive charge on the acyl carbon atom, which is therefore necessary for nucleophilic substitution and also the intermediate compound is also stabilized.
Complete Step By Step Answer:
Hoffmann Bromamide Degradation reaction is a reaction in which an amide is treated with bromine in the presence of an alkali as a strong base that attacks the amide and leads to the deprotonation and subsequent generation of an anion. Hoffmann Bromamide Degradation reaction is used to convert primary amide into primary amine.
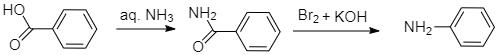
For the compound (Y) when benzoic acid is treated with ammonia, ammonium benzoate is formed which on further heating loses a molecule of water to form benzamide. Hence, benzoic acid when heated and treated with ammonia gives benzamide. Now, benzamide on treatment with bromine and KOH which is an alkali will give aniline with molecular formulaC6H5NH2. Therefore, the compound (Y) is aniline.
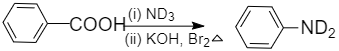
Similarly, for compound(X) when benzoic acid is treated with deuterated ammonia, C6H5COND2is formed which on further reaction with bromine and potassium hydroxide gives C6H5ND2as product.
Therefore the correct answer is option (2).
Note :
Amines are organic compounds and functional groups which contain a basic nitrogen atom with a lone pair and are derived from ammonia by the replacement of one or more hydrogen atoms by organic groups or substituents such as alkyl or aryl groups. A primary amine has one alkyl or aryl group on nitrogen whereas a secondary amine has two alkyl or aryl groups on nitrogen and tertiary amine has three alkyl or aryl groups on nitrogen.
