Question
Question: What are \[X\] and \[Y\] ? 
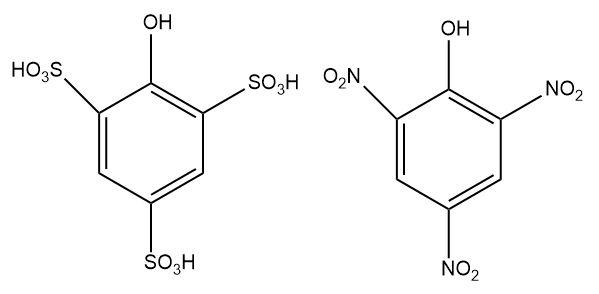
1.
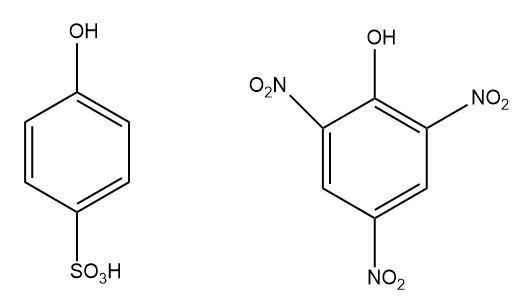
2.
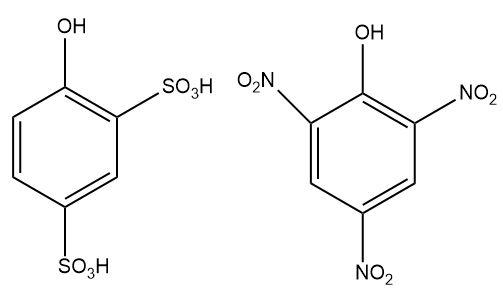
3.
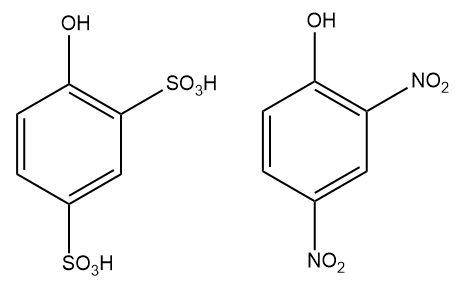
4.
Solution
Aromatic rings like benzene are a rich source of electrons and are readily attacked by electrophiles. The hydroxyl group attached to benzene results in the formation of phenol that further activates the ring towards electrophilic substitution reactions and is attacked at ortho and para position only.
Complete answer:
The hydroxyl group attached to the benzene ring in phenol has a strong electron donating effect (+M) called the mesomeric effect that strongly activates the benzene ring and makes it susceptible to attack by electrophiles. The electron density of the phenol ring is enhanced at specific positions called the ortho and para position.
Concentrated sulphuric acid is used as a sulphonating agent that selectively attacks the ortho and para positions. But the attack at para position is preferred more than that of ortho position due to the formation of a thermodynamic product. Therefore, sulphonation reaction results in the formation of para substituted phenol.
Further, reaction with concentrated nitric acid on a highly activated para sulfonated phenol ring results in the displacement of (−SO3H) by the nitro group at the para position along with the attack at ortho positions. Thus a vigorous nitration reaction results in the formation of a phenol derivative with three nitro groups (NO2) occupying the ortho and para position of phenol and this compound is known as picric acid.
The reaction can be written as follows:
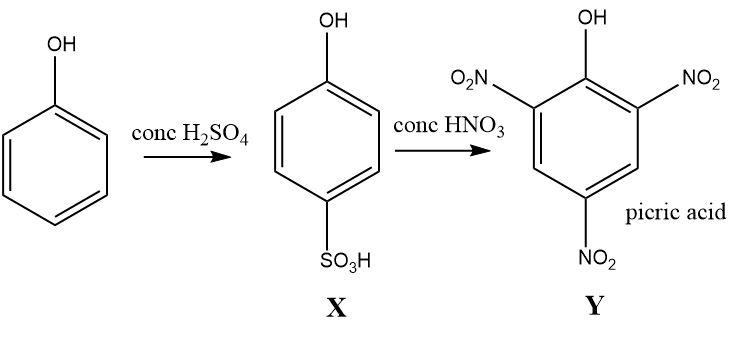
Hence, the correct option is (2).
Note:
Simple nitration of phenols in the absence of concentrated sulphuric acid is always carried out with the help of dilute nitric acid. Concentrated nitric acid is a strong oxidizing agent that can oxidize phenols and prevent the formation of the nitration product.
