Question
Question: What are two conformations of cis-1,4-dimethyl cyclohexane?...
What are two conformations of cis-1,4-dimethyl cyclohexane?
Solution
Hint : Just two stereoisomers, cis- and trans- 1,4-dimethyl cyclohexane, have an internal symmetry plane. As a result, both are meso compounds (optically inactive). Only cis-1,3-dimethyl cyclohexane, unlike 1,4-dimethyl cyclohexane, has an internal symmetry plane, whereas trans-1,3-dimethyl cyclohexane does not.
Complete Step By Step Answer:
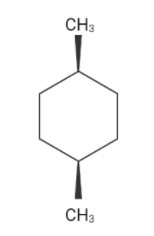
Cis-1,4-dimethyl cyclohexane has one chair conformation and two boat conformations. The chair conformation of cyclohexane is more stable than the boat conformation since the C−H bonds are similarly axial and equatorial in the chair conformation, i.e., six of the twelve C−Hbonds are axial and six are equatorial, and each carbon has one axial and one equatorial C−Hbond.
The chain structure of cis-1,4-dimethyl cyclohexane
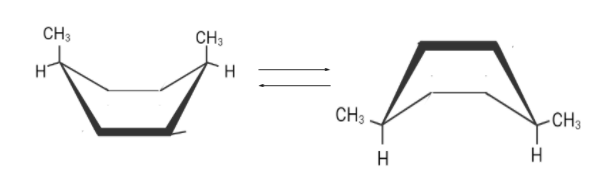
There are two flipped boat conformations . Although both are different but the right one is more stable.
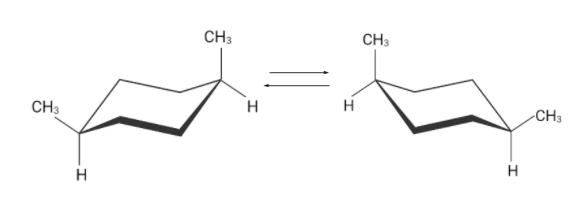
Similarly there are two flipped cyclohexane chairs. These two conformations are identical.
Note :
This compound can be used in various real life applications. The absorption of ultrasonic waves that can be calculated using a reverberation method has been studied using cis-1,4-dimethylcyclohexane.
