Question
Question: What are the structures of metaphosphoric acid and trimetaphosphoric acid?...
What are the structures of metaphosphoric acid and trimetaphosphoric acid?
Solution
Metaphosphoric acid and trimetaphosphoric acid are the oxoacids of phosphorus. Phosphorus forms a double bond with the O atom and forms a single bond with the OH group.
Complete answer:
Metaphosphoric acid: It is the oxoacid of phosphorus which has a formula HPO3.
Metaphosphoric acid has three forms: i) single-molecule form, ii) trimetaphosphoric acid which has a cyclo structure, iii) poly metaphosphoric acid which has a linear polymer chain structure.
In metaphosphoric acid, the phosphorus is in the +5 oxidation state.
In the structure, it forms two double bonds with the oxygen atom and one single bond with the OH group.
The structure of metaphosphoric acid is given below:
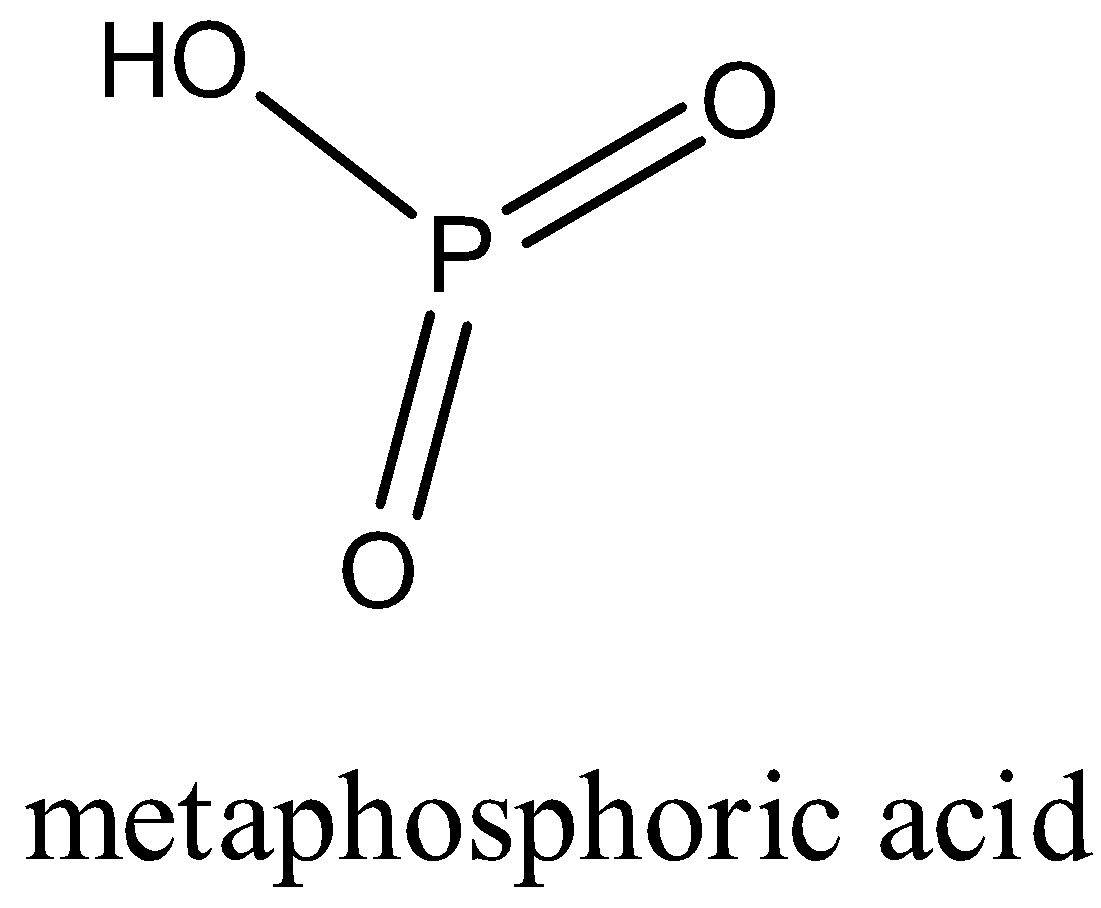
It is a monobasic compound.
Trimetaphosphoric acid: It is also an oxoacid of phosphorus which has a formula (HPO3)3.
It is commonly called cyclotrimetaphosphoric acid because they form a cyclic structure. The three metaphosphoric acid molecules form a ring structure.
In trimetaphosphoric acid, the phosphorus is in the +5 oxidation state.
In this structure, there are three P-OH bonds, three P=O bonds, and three P-O-P bonds.
The structure of trimetaphosphoric acid is given below:
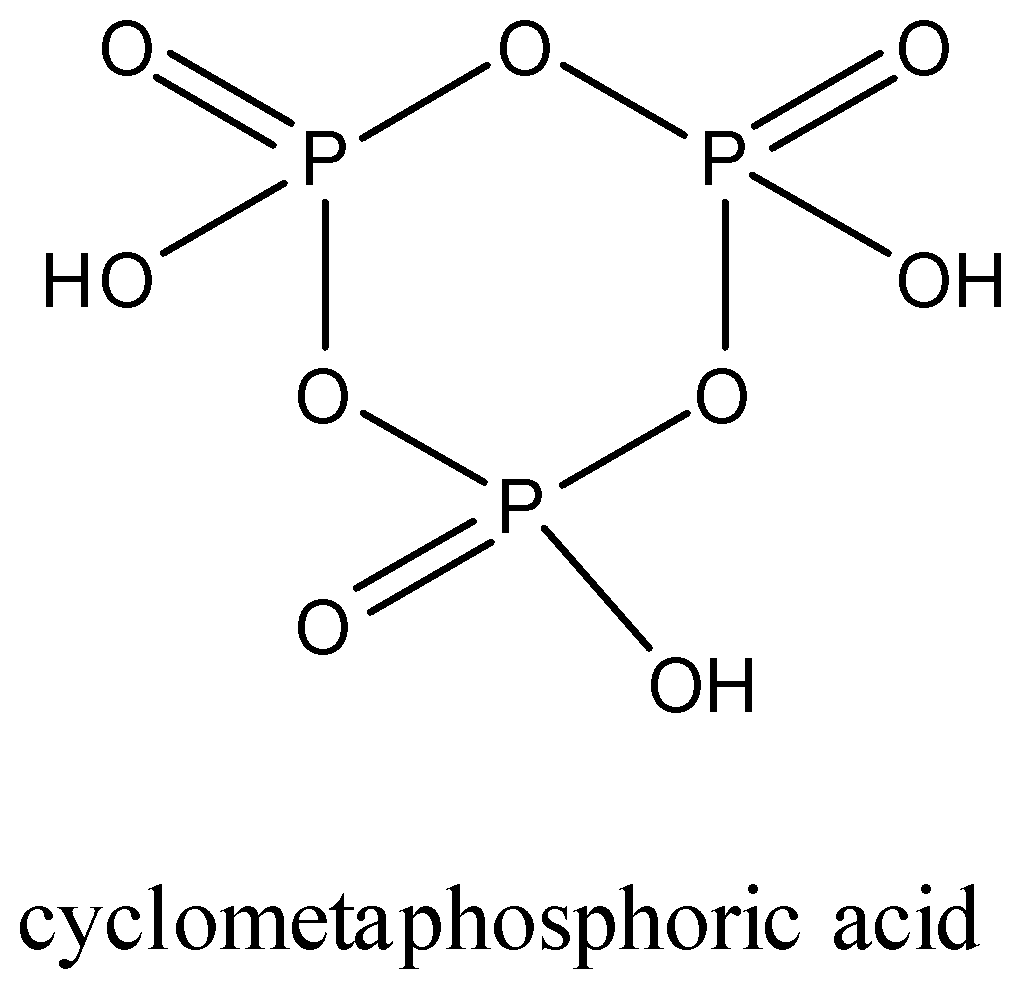
It is a tribasic compound.
Additional Information:
There is one more form of metaphosphoric acid, which is called poly metaphosphoric acid. The formula of poly metaphosphoric acid is (HPO3)n.
It has a linear polymer structure. The oxidation state of phosphorus is +5.
The structure of poly metaphosphoric acid is given below:
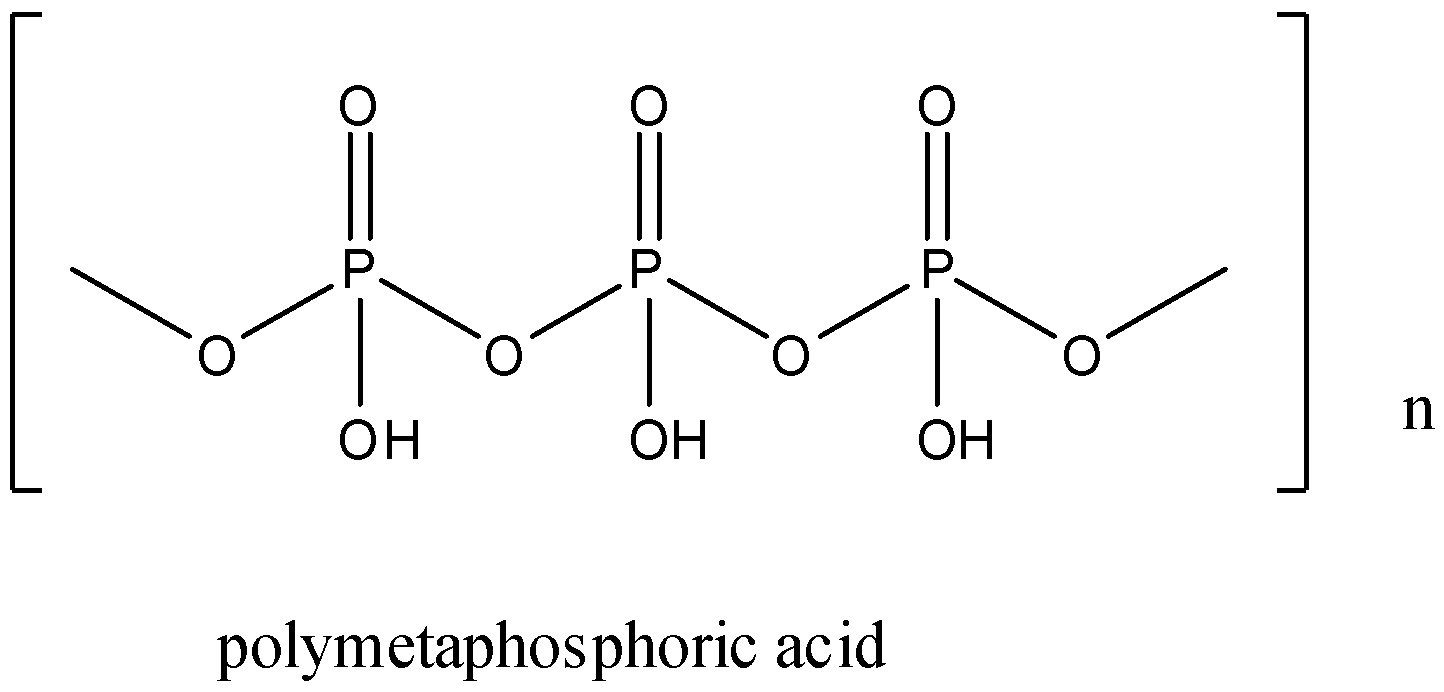
Note: According to the structure of the oxoacids of phosphorus we can find the basicity of the compound. The number of OH groups in the compound is equal to the basicity of the compound.
