Question
Question: What are the steps used to draw Lewis structure?...
What are the steps used to draw Lewis structure?
Solution
Lewis structure is a very easy useful method or structure in which we can find the number of valence electrons present in the atom and the number and type of bonds formed between the atoms in the compound.
Complete answer:
The number of bonds and types of bonds is represented by many structures, so Lewis structure is one of the important methods to determine the structure. Lewis structure is a very easy useful method or structure in which we can find the number of valence electrons present in the atom and the number and type of bonds formed between the atoms in the compound.
The steps involved are:
Write the symbol of the element, then, find the number of valence electrons in the valence shell of the element, then, draw the bonds by either transfer or sharing of two electrons. These two electrons must be shared by both the elements. Then find the total number of valence electrons in the compound and balance it in such a form that each element can complete its octet rule.
Let us understand this with an example:
The ammonia compound has 4 atoms, three hydrogen atoms, and 1 nitrogen atom.
The number of valence electrons in a nitrogen atom is 5 and there are three hydrogen atoms around the nitrogen atom and each hydrogen atom will form a single bond as:
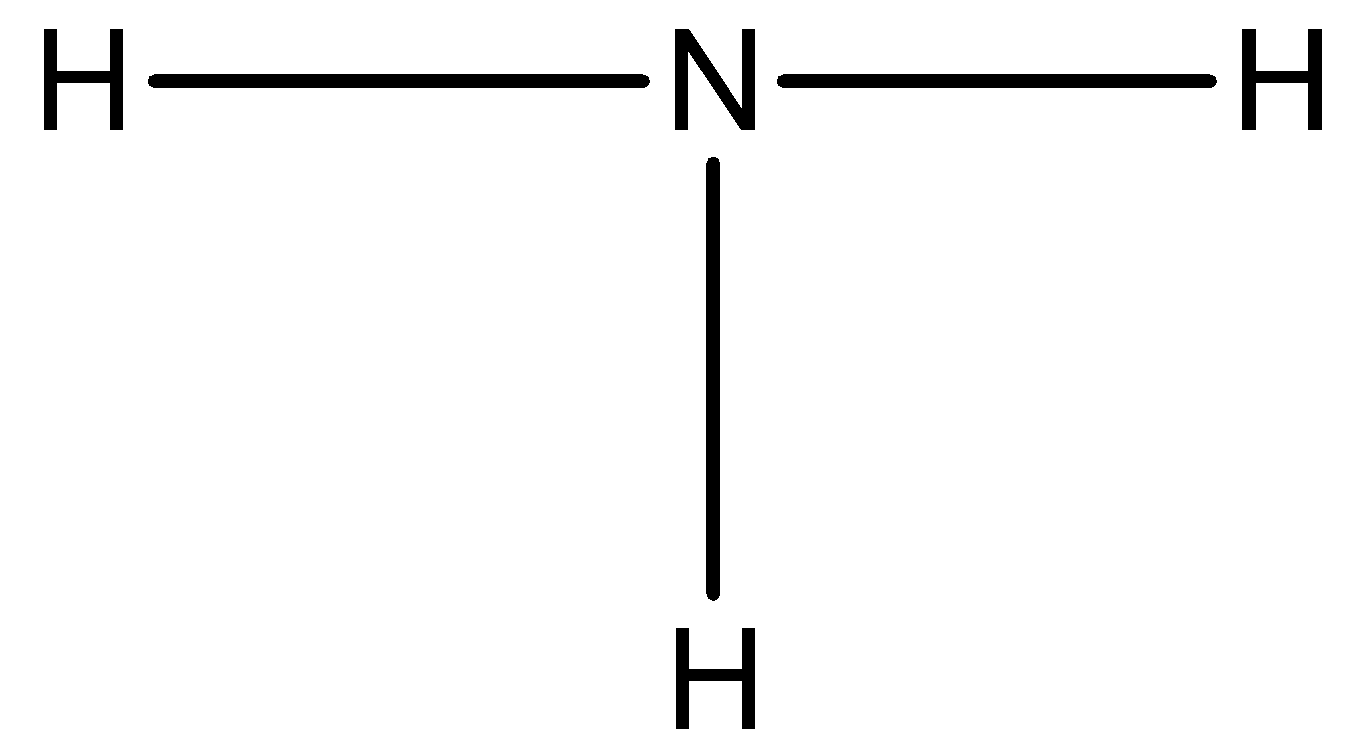
Now, three of the valence electrons are used, so now we can draw a lone pair of electrons on the nitrogen atom.
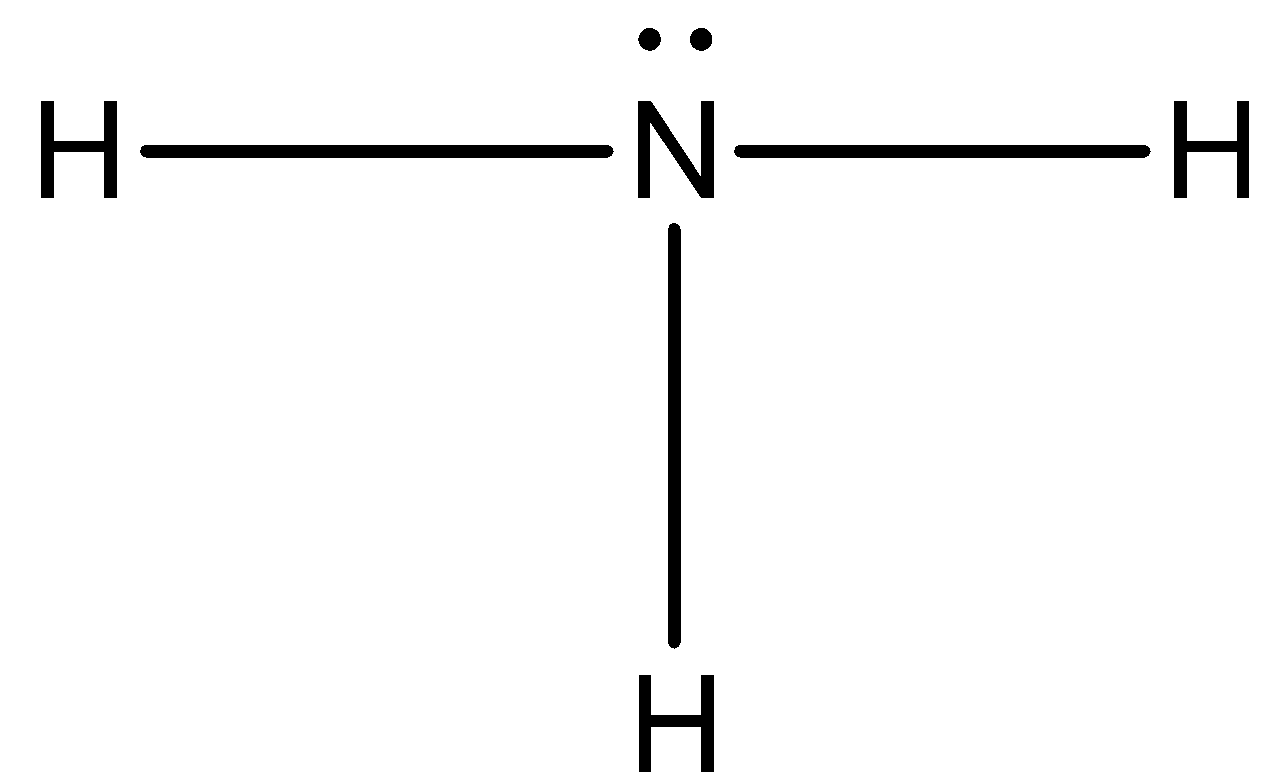
The octet of nitrogen has completed and each hydrogen atom has two electrons.
Note:
Only the hydrogen and helium atom doesn't need eight electrons because they have only 1s orbital and they can have a maximum of 2 electrons in its orbital, the rest of all the elements want to complete its octet.
