Question
Question: What are the steps associated with the process of constructing a hybrid orbital diagram?...
What are the steps associated with the process of constructing a hybrid orbital diagram?
Solution
The hybrid orbital diagram describes and shows the sigma and pi bonding present in a compound. With the help of this diagram the number of sigma and pi bonds can be calculated. To draw the hybrid orbital diagram, we need to know the hybridisation of the central atom present.
Complete answer:
We will take a molecule for reference and know the process of constructing a hybrid orbital diagram.
Consider the Ethylene molecule, also known as Ethene. It has two carbon atoms with a double bond present in between the two carbons. The molecular formula is H2C=CH2. The steps of constructing a hybrid orbital diagram are as follows:
Step 1: Draw the Lewis structure of the ethene molecule.
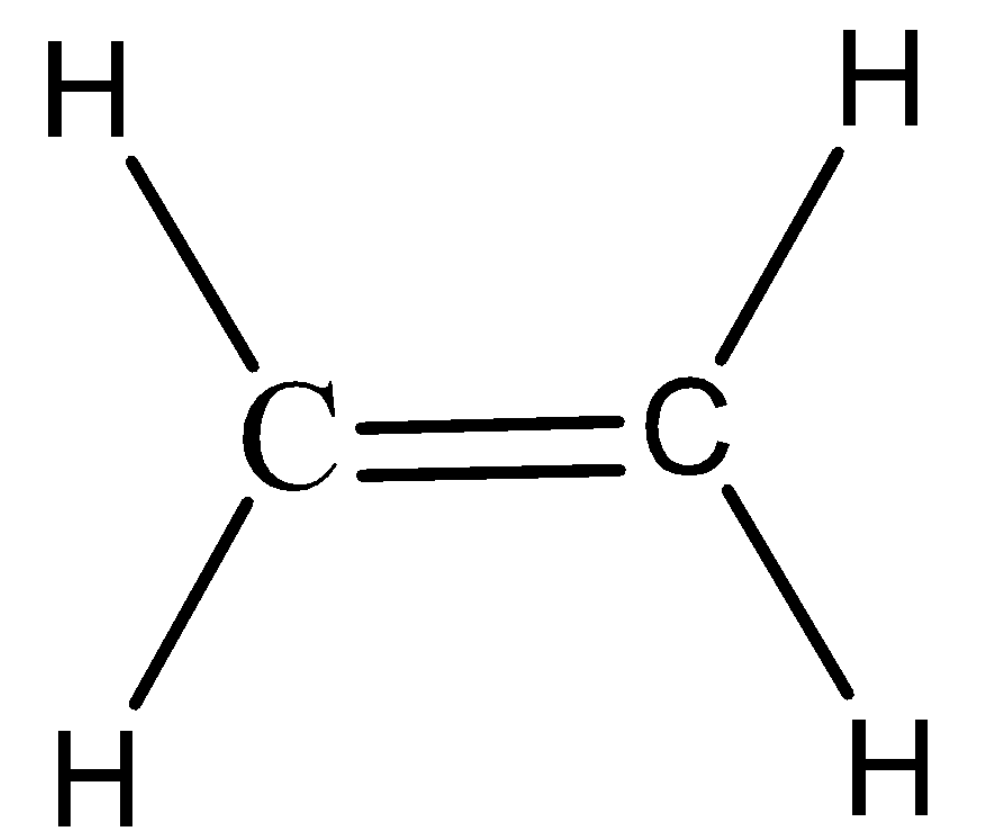
Step 2: We will determine the geometry around each central atom i.e. Carbon using the VSEPR theory. Here the carbon is attached to a double bond and two hydrogens. Therefore, we can say that it has a AX3 system , hence the geometry is Trigonal Planar.
Step 3: Determine the hybridization of the central atom using the geometry. Here the geometry is Trigonal Planar, hence the hybridisation will be sp2 . Each carbon atom in the ethene molecule has a sp2 hybridisation.
Step 4. Draw the two Carbon atoms side by side of each other and bring the next carbon and Hydrogen close to each other, such that they can overlap to form sigma and pi bonds. The sigma and pi bonding of ethene can be shown below:
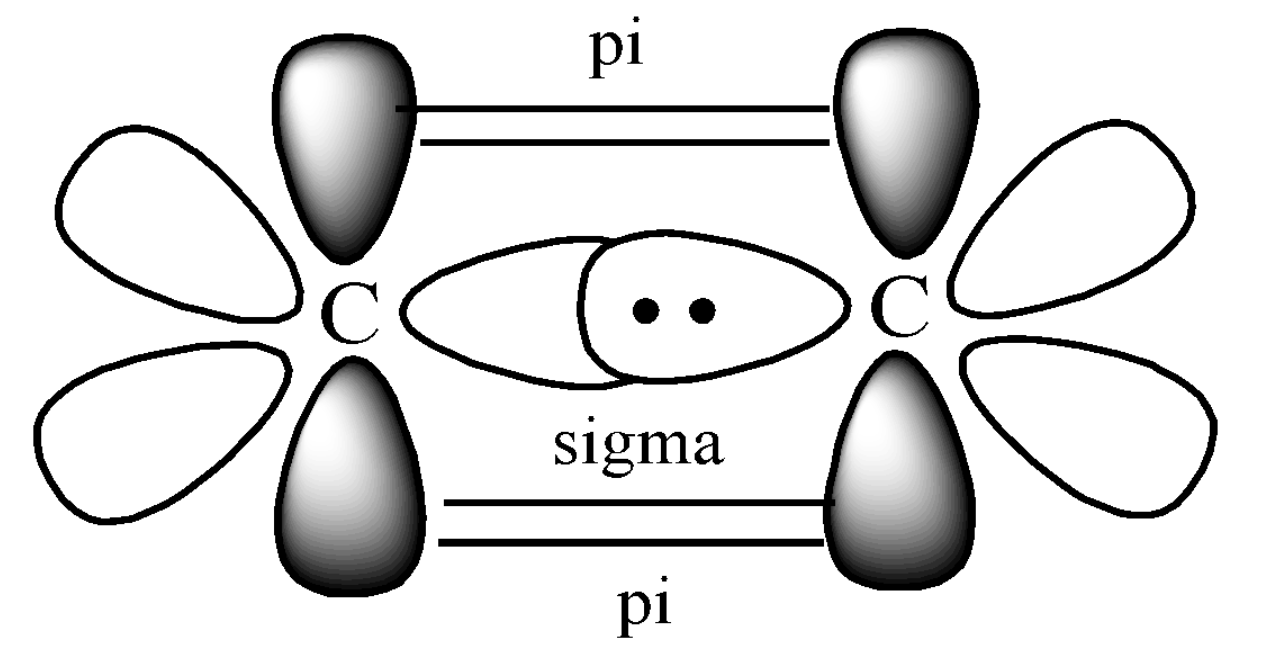
Here, the C-C head-on collision forms the sigma bond and the perpendicular overlap of the p orbitals forms the pi bond. Hence, we can say that ethylene has one sigma and one pi bond present between the two Carbon atoms. The perpendicular p-orbital will always form a pi bond, whereas the orbital in the plane will form a sigma bond only. This is the hybrid orbital diagram of ethene. We follow the same process for any molecule.
Note:
Sigma bonds are formed due to head-to-head collisions of orbitals that reside in the same plane. Pi bonds are formed by lateral overlap of orbitals which are perpendicular to the plane. Sigma bonds are stronger than pi bonds because of this reason only.
