Question
Question: What are the shapes of the molecules of water and boron trifluoride? | \({{H}_{2}}O\) | \(B...
What are the shapes of the molecules of water and boron trifluoride?
| H2O | BF3
---|---|---
A| Linear| Pyramidal
B| Linear| Trigonal
C| Non-linear| Pyramidal
D| Non-linear| trigonal
(a) A
(b) B
(c) C
(d) D
Solution
By calculating the by hybridization of H2O and BF3 (it is process of inter-mixing of the orbitals to form new orbitals of equivalent energy),we can know about their shapes. The hybridization can be calculated by the formula:
H= 21 (V+M-C+A)
Here, V represents the number of electrons in the valence shell of the atom, M represents the monovalent atoms attached to that atom and C and A represents the charges of the cations and anions. Now, Identify the correct statement.
Complete answer:
First of all, let’s discuss what hybridization is. By the term hybridization we mean the phenomenon of inter-mixing of the orbitals of slightly different energies so as to redistribute their energies and to give a new set of orbitals of equivalent energies and shape.
First, we have to find the hybridization of PF5 molecule by the formula as: H= 21 (V+M-C+A) -(1)
Here, V= number of the valence electrons in the atom, M= number of the monovalent atoms bonded to the central atom, C=the charge on the cation and A= the charge on the anion.
Now, calculating the hybridization of both H2O and BF3by using this formula as;
In H2O ;
Number of valence electrons in oxygen =6
Number of monovalent electrons=2
Then, we get;
Hybridization of H2O=21(6+2)=21×8=4
Since, its hybridization is sp3, therefore, it has pyramidal geometry due to the presence of two lone pairs of electrons and two bond pairs instead of the tetrahedral geometry and thus, has non-linear shape as;

Similarly, in BF3;
Number of valence electrons in B=3
Number of monovalent atoms=3
Then, we get;
Hybridization of BF3 =21(3+3)=21×6=3 =3
Since, its hybridization is sp2, therefore, it has trigonal planar geometry due to the presence of three bond pairs as;
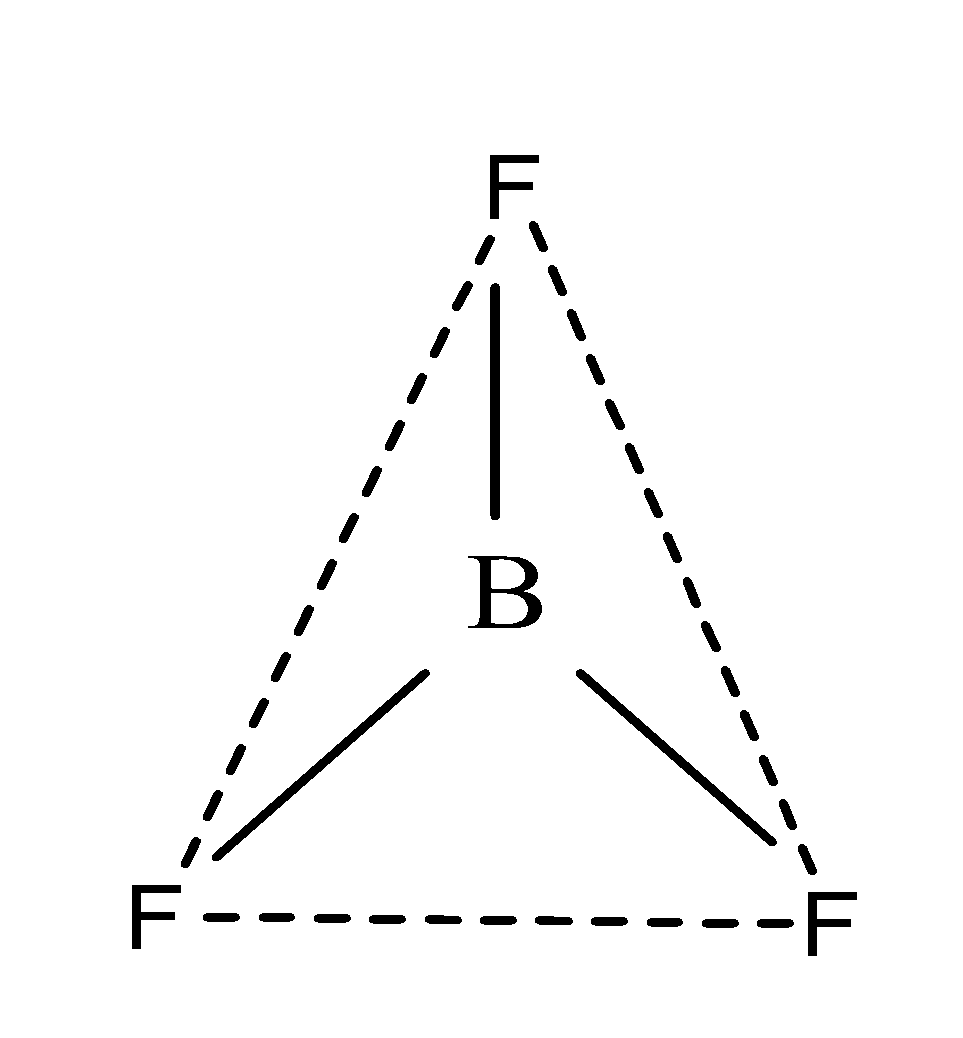
So, the shapes of the molecules of water and boron trifluoride are non- linear and trigonal respectively.
Hence, option(d) is correct.
Note: Hybridization helps us to predict the shape, geometry and structure of the molecules and isostructural are those structures which have the same number of atoms and they are arranged in the same way in the similar structures.
