Question
Question: What are the shapes of \(PC{l_4}^ + \) , \(PC{l_4}^ - \) and \(AsC{l_5}\) ? A. Square planar, tetr...
What are the shapes of PCl4+ , PCl4− and AsCl5 ?
A. Square planar, tetrahedral and see-saw
B. Tetrahedral, see-saw and trigonal bipyramidal
C. Tetrahedral, square planar and pentagonal bipyramidal
D. Trigonal bipyramidal, tetrahedral and square
Solution
With the valency of the central atom, find out the hybridization of the molecule. The hybridization of the molecule determines the shape of the molecule. We should also check for the presence of lone pairs and the change in shape due to that.
Complete step-by-step answer: I) PCl4+ : In this molecule, the valency of central atom Phosphorus is 5 and due to the positive charge one electron is removed. So now the remaining 4 electrons bond with one Chlorine each.
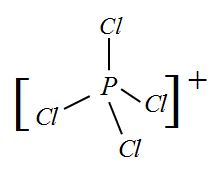
Since there are 4 bonds, the hybridization will be sp3 and the shape corresponding to this hybridization is Tetrahedral.
II) PCl4− : The valency of Phosphorus is 5 and given a negative charge so now we will have 4 electrons bonded to Chlorine and one lone pair. Hence, the hybridization would be sp3d . Since we have a lone pair, the hybridization changes from sp3 to sp3d .
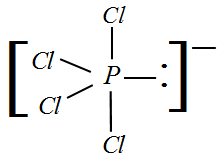
Therefore, for a molecule with a lone pair and sp3d hybridization, the shape would be see-saw.
III) AsCl5 : Since Arsenic belongs to the same group as Phosphorus, we can write the valency of Arsenic to be 5. All the 5 electrons are bonded with one Chlorine each and hence we can deduce the hybridization to be sp3d and there are no lone pairs.
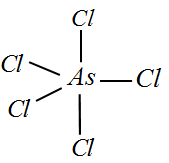
Therefore the shape would be trigonal bipyramidal.
Additional information: Hybridization of a molecule plays an important role in determining the shape of that molecule. Presence of a positive or negative charge or a lone pair changes the shape of the molecule.
Note: If the hybridization of the given molecule is sp3 then it’s shape would be tetragonal. If the hybridization is sp3d without a lone pair then the shape would be trigonal bipyramidal and with a lone pair the shape would be see-saw.
