Question
Question: What are the set of d orbitals involved in forming capped octahedral geometry?...
What are the set of d orbitals involved in forming capped octahedral geometry?
Solution
An atomic orbital is a mathematical function in atomic theory and quantum physics that describes the position and wave-like behaviour of an electron in an atom. This function may be used to determine the likelihood of locating any atom's electron in any given area surrounding the nucleus. The phrase atomic orbital can also refer to the actual region or space in which the electron can be calculated to be present, based on the orbital's mathematical structure.
Complete answer:
The form of compounds containing six atoms or groups of atoms or ligands symmetrically grouped around a central atom, defining the vertices of an octahedron, is described by octahedral molecular geometry in chemistry. The prefix octa comes from the fact that the octahedron has eight faces. Although octahedral compounds generally contain a single atom in the centre and no links between the ligand atoms, the octahedron is one of the Platonic solids.
The capped octahedral molecular geometry in chemistry defines the structure of compounds with seven atoms or groups of atoms or ligands grouped around a central atom, defining the vertices of a gyroelongated triangular pyramid. This form, along with the pentagonal bipyramid and the capped trigonal prism, has C3v symmetry and is one of three typical geometries for heptacoordinate transition metal complexes.
The ions heptafluoro molybdate (MoF7−) and heptafluoro tungstate (WF7−) both have a capped octahedral molecular shape.
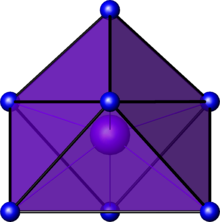
A capped octahedral geometry is octahedral with an additional ligand above the equatorial plane, in between the equatorial ligands. The primary axis of rotation is the C3(z) axis, which belongs to the C3v point group. Another perspective is to look down the C3(z) axis.
The dz2 points to the cap atom since the z axis passes through it. Because the atoms on the octahedral face (which form the triangle in the second image) are on the xy plane, we require both on-axis and off-axis d orbitals (dx2−y2 and dxy) to explain this hybridization.
As a result, one possibility that comes to mind is dz2,dx2−y2,dxy.
Note:
Alfred Werner established the idea of octahedral coordination geometry to explain stoichiometries and isomerism in coordination compounds. Chemists were able to rationalise the number of isomers of coordination compounds because of his understanding. Werner-type transition-metal complexes are octahedral transition-metal complexes with amines and simple anions.
