Question
Question: What are the products of the following anti-Markovnikov reaction: \[C{H_3} - C(C{H_3}) = CH - C{H_...
What are the products of the following anti-Markovnikov reaction:
CH3−C(CH3)=CH−CH3+HBr+H2O2?
Solution
Free-radical addition reaction: It is a chemical reaction of alkenes in which a free radical acts as an intermediate. The addition can be performed between a radical and a non-radical molecule or between two radicals. Anti-Markovnikov’s reaction is an example of a free-radical addition reaction.
Complete answer:
Anti-Markovnikov’s Rule: When an unsaturated hydrocarbon reacts with hydrogen bromide i.e., HBr in the presence of hydrogen peroxide i.e., H2O2, then the bromide ion will be bonded to the least substituted unsaturated carbon atom. It is also known as the peroxide effect. It follows a free radical mechanism to form the product.
The reaction mechanism for given hydrocarbon to undergo Anti-Markovnikov reaction is as follows:
Step-1: Initiation:
Hydrogen peroxide is an unstable molecule, so in the presence of heat or sunlight the homolytic cleavage of H2O2 molecules takes place and respective radicals are formed. The reaction is as follows:

The free radical reacts with hydrogen bromide to form bromine radical. The probability of formation of hydrogen radical is very less due to high instability. So, formation of only bromine radical takes place. The reaction is as follows:

Step-2: Propagation:
The bromine radical reacts with the less substituted unsaturated carbon atom to form a more stable free radical. The reaction is as follows:

Then the radical extracts hydrogen radical from hydrogen bromide and the final product is formed along with the removal of bromine radical. The reaction is as follows:
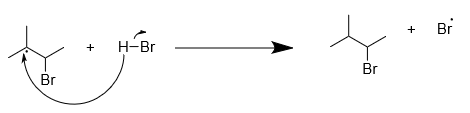
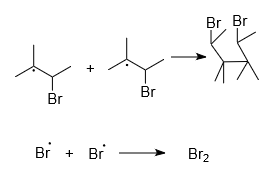
Step-3: Termination
It is the step in which no radical formation takes place as a product. Only minor or side products are formed in this step. Side products formed are as shown below:
Overall Anti-Markovnikov reaction for the given unsaturated hydrocarbon is as follows:
CH3−C(CH3)=CH−CH3+HBr+H2O2→CH3−C(CH3)−CH(Br)−CH3
Hence the final product is 2−bromo,2−methylbutane.
Note:
Important points to remember regarding the anti-Markovnikov reaction.
-The alkene must be asymmetrical i.e., there must be different groups attached to each unsaturated carbon atom.
-Anti-Markovnikov products are only formed in the presence of hydrogen bromide i.e., HBr. For any other hydrogen halide, only Markovnikov products will be formed.
-A peroxide group must be present in the reaction mechanism.
