Question
Question: What are the products obtained upon the ozonolysis of 2-pentene? (This question has multiple correct...
What are the products obtained upon the ozonolysis of 2-pentene? (This question has multiple correct answers)
(a)- CH3CH2CHO
(b)- CH3CHO
(c)- CH3COCH3
(d)- CH3COCH2CH3
Solution
Ozonolysis means the given organic compound will be treated with O3 molecules. When an alkene is treated with ozone, then the double bond will break and all the three oxygen atoms will form a ring structure around the double bond and further on treatment with ZnO carbonyl compounds will form.
Complete step-by-step answer: Ozonolysis means the given organic compound will be treated with O3 molecules. When an alkene is treated with ozone, then the double bond will break and all the three oxygen atoms will form a ring structure around the double bond and further on treatment with ZnO carbonyl compounds will form.
2-pentene is an alkene of five carbon atoms and the double bond is present between the second and third carbon atoms. The formula is given below:
CH3−CH=CH−CH2−CH3
Now, when the 2-pentene is treated with ozone, the double bond will break and a ring structure will be formed. The reaction is given below:

Now, this ring structure formed is called pent-2-ene ozonide. This molecule is reduced with zinc oxide (ZnO), in which the oxygen atom on the top will be removed, and rest two oxygen bonds will break and form two types of aldehyde, i.e., acetaldehyde and propanal. Their formula will be CH3CHO and CH3CH2CHO. The reaction is given below:
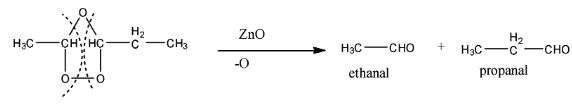
Therefore, the correct answers will be options (a) and option (b).
Note: If the given alkene has only a single chain then all the products formed will be aldehydes but there are branched structures in the alkene, there could be the formation of aldehydes and ketones as well.
