Question
Question: What are the products formed in the given reaction? 
A.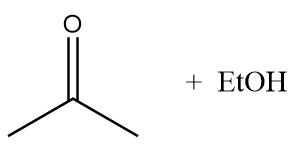
B.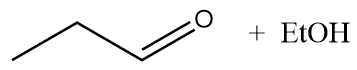
C.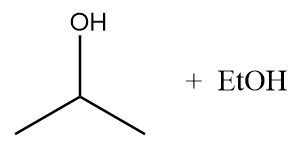
D.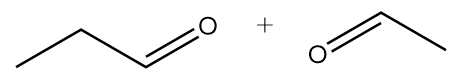
Solution
Hint : When ethers are reacted to a nucleophile in the presence of strong acid, then their bond cleaves to give respective alcohols and other compounds that depend on the type of nucleophile considered. The mechanism involved in the process in bimolecular nucleophilic substitution reaction.
Complete Step By Step Answer:
The reaction proceeds in certain steps which are given as follows:
Step-1: Protonation of ether.
The hydrogen ion present in the medium attacks the lone pair of electrons of the oxygen atom due to which formation of a protonated compound takes place. The reaction proceeds as follows:
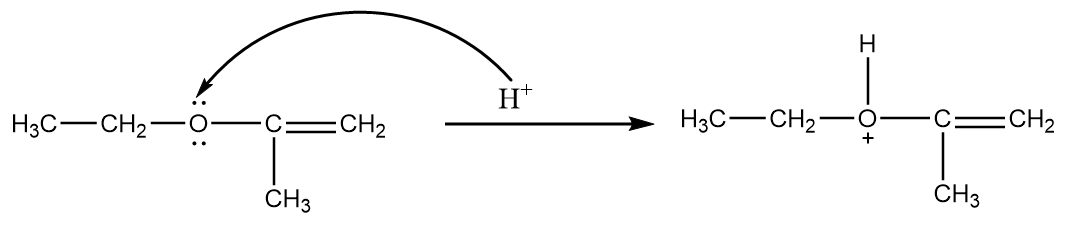
Step-2: attack of nucleophile via SN2 reaction mechanism.
The hydroxide ion of the water molecule will act as a nucleophile and replace the alcohol group from the compound. The reaction takes place as follows:
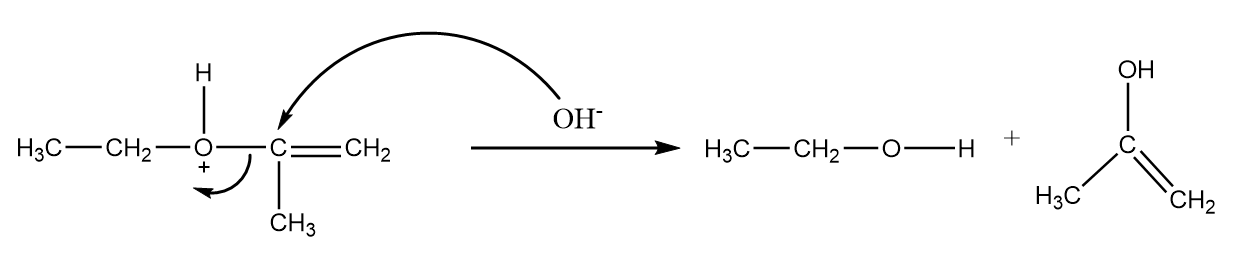
Step-3: Keto-enol tautomerism.
The unsaturated alcohol will undergo keto-enol tautomerism to form acetone. The reaction proceeds as follows:
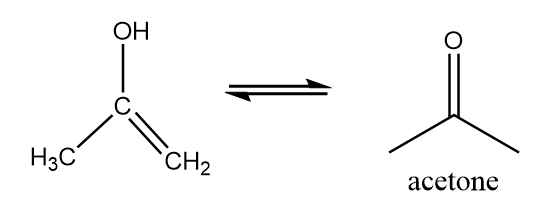
Hence, final products formed after the reaction are given below:
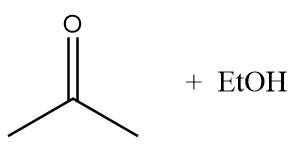
Therefore, option (A) is the correct answer.
Note :
It is important to note that, because of the acidity of alpha hydrogens, carbonyl compounds undergo a proton transfer equilibrium termed as tautomerism. In keto-enol tautomerism, the keto tautomer is preferred because of its structure. The ketone consists of two alkyl groups which shows +I effect and donate electron density to the carbon atom at the carbonyl centre and thus lead to a more stable compound. Also, the carbonyl bond i.e., C=O bond is much stronger than C=C bond and hence, the unsaturated alcohols generally exist as keto tautomers.
