Question
Question: What are the possible resonance structures of \( C{H_3} - \,CH\, = \,CH\, - \,CH\, = \,O \) and \( C...
What are the possible resonance structures of CH3−CH=CH−CH=O and CH3−COO− ? What is the order of stability of the contributing resonance structures?
Solution
Hint : A group of two or more Lewis Structures that collectively explain the electronic bonding of a single polyatomic species, including fractional bonds and fractional charges, is known as a resonance structure.
Complete Step By Step Answer:
Remember that the electrons will pass to the more electronegative or positive atom.
(a)But-2en-al
2-Butenal, also known as crotonaldehyde, is a member of the enal family of organic compounds. Since there is no such thing as a positive atom, but because O is the most electronegative, let us transfer electrons to it.
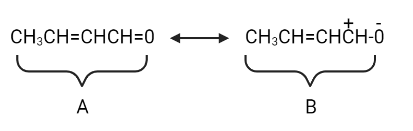
Let's move electrons to the carbon atom now that it has a positive charge.
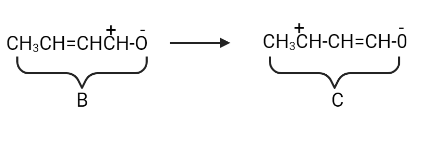
Structure A contributes the most and has the lowest energy. Structures B and C have a higher energy level and contribute less.
(b) Acetate ion
The removal of a proton from the carboxyl group of acetic acid produces acetate, a monocarboxylic acid anion.
There is no positive atom in this case, but the other O atom is an electronegative atom. Let us transfer the electrons in that direction.
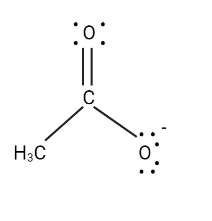
To get the third structure shown below, transfer the electrons in the carbonyl group to the carbonyl O atom.
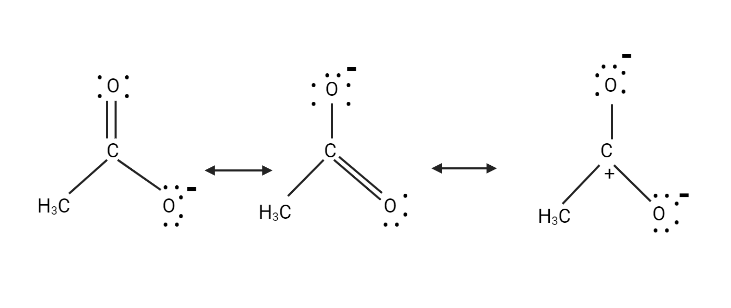
Of the three structures above, the first two structures are similar and have the same amount of energy. Since the third structure has three charges instead of one, it is just a minor contributor.
Note :
The basic aim of resonance structures is to demonstrate that molecules can transfer electrons and charges to different atoms. Since the charge (or bond) is now delocalized and not "forced" onto an atom that does not want it, resonance makes a molecule more stable.
