Question
Question: What are the oxidation numbers of the underlined elements in each of the following and how do you ra...
What are the oxidation numbers of the underlined elements in each of the following and how do you rationalize your results?
(a) Fe3O4
Solution
The oxidation state, also known as the oxidation number, describes the degree of oxidation (electronic loss) of an atom in a chemical compound. The oxidation state, which can be positive, negative, or zero in concept, is the hypothetical charge that an atom would have if all bonds to atoms of different elements were entirely ionic, with no covalent component. This is never entirely accurate for real bonds.
Complete answer:
When the Oxidation Number of O is taken as+2, the number of Fe is found to be. +2×32 =34= Oxidation Number ., on the other hand, it cannot be fractional. In this case, one of the three numbers of Fe atoms have an Oxidation Number of +2, while the other two numbers of Fe atoms have an Oxidation Number of +3.
The oxidation number of Fe in FeO is +2,
and the oxidation number of Fe in Fe2O3+3
An atom's oxidation number is a number that represents the total number of electrons it has lost or gained.
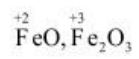
Calculating Oxidation Number
The following rules can be used to assign an oxidation number to a given element or compound.
-Any free element has an oxidation number equal to zero.
-For monatomic ions, the oxidation number always has the same value as the net charge corresponding to the ion.
-The hydrogen atom (H) exhibits an oxidation state of +1
-However, when bonded with an element with less electronegativity than it, it exhibits an oxidation number of −1.
-Oxygen has an oxidation of −2 in most of its compounds. However, in the case of peroxides, the oxidation number corresponding to oxygen is −1. .
-All alkali metals (group 1 elements) have an oxidation state of +1 in their compounds.
Note:
When polyatomic ions are considered, the sum of all the oxidation numbers of the atoms that constitute them equals the net charge of the polyatomic ion. Thus, the oxidation number of an atom in a given compound can be calculated with the steps mentioned above.
