Question
Question: What are the oxidation numbers of the underlined elements in each of the following and how do you ra...
What are the oxidation numbers of the underlined elements in each of the following and how do you rationalize your result?
a. KI3
b. H2S4O6
c. Fe3O4
d. CH3CH2OH
e. CH3COOH
Solution
In simple words, the oxidation number is the number assigned to the components in a chemical combination. The oxidation number is the total number of electrons that atoms in a molecule can share, lose, or gain while forming chemical interactions with atoms of another element.
Complete answer:
(a) KI3
The oxidation number (O.N.) of K in KI3 is +1 . As a result, average oxidation number of I is −31 . O.N., on the other hand, cannot be fractional. To determine the oxidation states, we must first consider the structure of KI3 . An iodine atom forms a coordinate covalent bond with an iodine molecule in a KI3 molecule.
K++1[I0−I0←I−1]−
As a result, the O.N. of the two I atoms that make up the I2 molecule in a KI3 molecule is 0, whereas the O.N. of the I atom that makes up the coordinate bond is −1.
(b) H2S4O6
2H+1SxO46O−2
Now,
2(+1)+4(x)+6(−2)=0 ⇒2+4x−12=0 ⇒x=+221
O.N., on the other hand, cannot be fractional. As a result, S in the molecule must exist in several oxidation states.
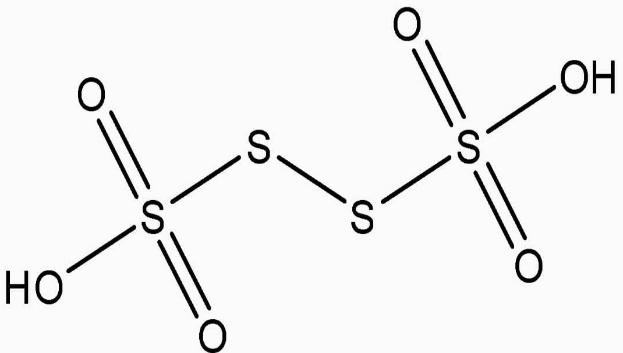
Two of the four S atoms have an O.N. of +5, whereas the other two have an O.N. of 0.
c. Fe3O4
The O.N. of Fe is determined to be when the O.N. of O is set to −2 . +2(32) O.N., on the other hand, cannot be fractional.
One of the three Fe atoms in this example has an O.N. of +2 , whereas the other two Fe atoms have an O.N. of +3 .
Fe+2O,Fe+3O3
d. CH3CH2OH
2Cx6H+12O−2
Hence, the O.N of C is −2 .
e. CH3COOH
2Cx4H+12O−2
2(x)+4(+1)+2(−2)=0 ⇒2x+4−4=0 ⇒x=0
The average O.N. of C , on the other hand, is 0. This molecule's two carbon atoms are found in two separate settings. As a result, their oxidation numbers cannot be the same. As a result, Cin CH3COOH has the oxidation states +2 and −2
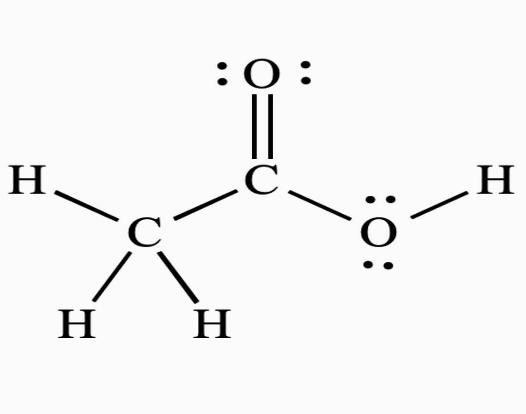
Note:
A negative oxidation state is attributed to the more electronegative element in a material. A positive oxidation state is attributed to the less electronegative element. Keep in mind that electronegativity is highest at the top-right corner of the periodic table and falls toward the bottom-left corner.
