Question
Question: What are the oxidation number of \(2C{l^ - }\) and \(C{l_2}\)...
What are the oxidation number of 2Cl− and Cl2
Solution
Hint : Oxidation number is the number of electrons gained or lost during the oxidation. If the given sign is positive it indicates electron loss. If the sign is negative it indicates electron gain.
Complete Step By Step Answer:
For 2Cl− iron it indicates that there are 2ions of Cl−present. That means the oxidation number is 2×(−1)=−2
Whereas for Cl2 it indicates 2atoms of Cl present. These to atoms share bonds to form chlorine gas.
There are total of 7 valance electrons per chlorine atom making it a total of 14in Cl2
These electron share a bond between 2chlorine atom to form Cl2.
One atom receives a charge of −1and the other receives a charge of +1 making the total of −1(+1)=0
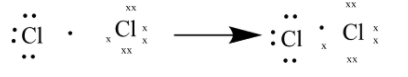
As seen in the above diagram each Cl atom shares one of the valance electrons to form a bond.
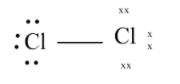
The final structure looks like
Therefore the oxidation number of Cl2is 0.
Note :
Chlorine gas is used in disinfection, paper producing industries etc. an atom having the highest electronegativity is given negative oxidation number.
