Question
Question: What are the number of bonding pairs and lone pairs of electrons in \({{H}_{2}}O\) ....
What are the number of bonding pairs and lone pairs of electrons in H2O .
Solution
The electrons which are going to involve in the formation of the bond between two atoms are called bond pairs and the electrons which are not involved in the bonding but present in the hybridized orbital are called lone pairs of electrons.
Complete answer:
- In the question it is given to write the number of bonding pairs and number of lone pairs of electrons in water molecules.
- To know about the number of bonding pairs and lone pairs of electrons present in water molecules, we should know the structure of the water molecule.
- The structure of the water molecule is as follows.
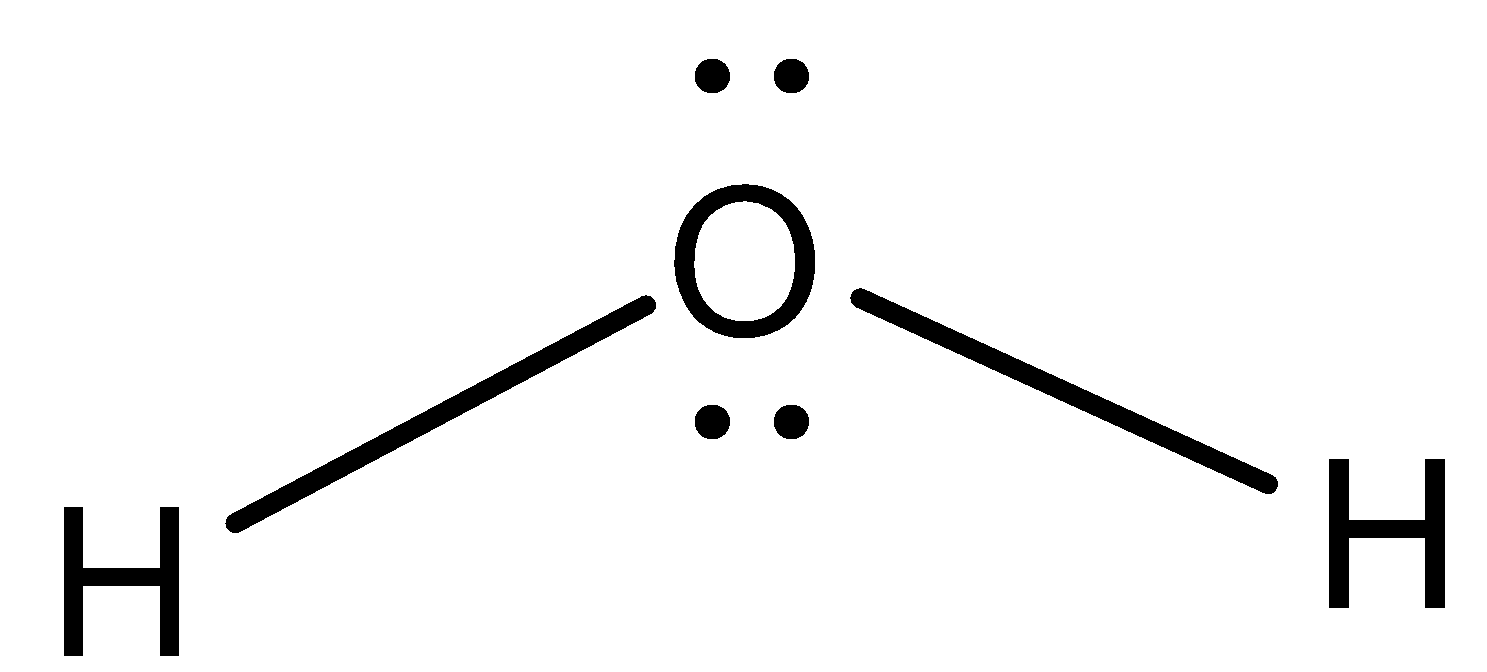
- From the structure we can easily say that there are two sigma bonds present in water molecules.
- The two hydrogens are bonded with oxygen through the sigma bonds.
- Means there are two bond pairs present in the water molecule.
- Coming to the concept of lone pair of electrons, there are two lone pairs of electrons which are located on the oxygen atom of the water molecule.
- Therefore the number of bond pairs and lone pairs present in water molecules are two.
Note:
Bond pair electrons are going to involve in bonding at the same time the lone pair electrons do not involve in bonding and they are going to label on the respective atom with two dots. Due to the presence of the lone pair of electrons there is a disturbance occurring in the structure of the molecule.
