Question
Question: What are the names of the elements in methane? A. Carbon and chlorine B. Carbon and hydrogen C...
What are the names of the elements in methane?
A. Carbon and chlorine
B. Carbon and hydrogen
C. Hydrogen and chlorine
D. Oxygen and hydrogen
Solution
In organic chemistry, an alkane which is also known as paraffin is an acyclic saturated hydrocarbon i.e., it consists of hydrogen and carbon atoms covalently bonded to each other via single bonds. The general chemical formula of alkanes is CnH2n+2.
Complete answer:
Methane is an organic compound with chemical formula CH4. It is the simplest alkane and main constituent of natural gas. It is a tetrahedral molecule with four equivalent C−H bonds. At room temperature and standard pressure, methane is a colourless and odourless gas. Structurally, it is represented as follows:
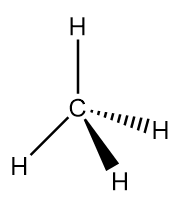
Thus, we can conclude the names of elements of methane are carbon and hydrogen.
Hence, option (B) is the correct answer.
Additional information-
The electronic configuration of a carbon atom is 1s22s22p2 that means only 2 electrons are unpaired in the ground state of the carbon atom. At an excited state, the mixing of orbitals takes place to generate hybrid orbitals with similar energy and electrons are rearranged in order to participate in bonding. This phenomenon is known as hybridization. In methane, the electrons are reorganized into one s and three p orbitals and thus, the carbon atom in methane is sp3 hybridized.
Note:
Remember that all the hydrogen atoms are bonded to the carbon atom via covalent bond i.e., the rotation of hydrogen atoms is not restricted. The hydrogen atoms in the methane molecule are allowed to rotate freely and thus, it is also referred to as a plastic crystal.
