Question
Question: What are the main intermolecular forces found in a liquid sample of \( CB{{r}_{4}} \) ?...
What are the main intermolecular forces found in a liquid sample of CBr4 ?
Solution
Hint : Intermolecular forces include all the types of forces of attraction as well as repulsion that are supposed to act between the atoms and with neighbouring particles of atoms like other atoms or ions. The different types of intermolecular forces acting are dispersion forces, dipole-dipole forces, hydrogen bonding, and ionic bonding.
Complete Step By Step Answer:
The theory of intermolecular forces was given by scientist Vander Waals. Hence, the intermolecular forces are known as the Vander Waals dispersion force, Vander Waals dipole-dipole interaction, and the hydrogen bond.
To understand the intermolecular forces, let us understand the structure of CBr4 which is as shown;
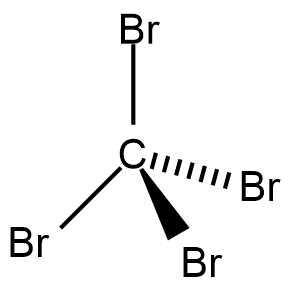
Let us consider the forces one by one.
The weakest intermolecular force is the Vander Waals dispersion forces (a temporary force that prevails upto a distance of 4.5A∘ ) which acts on the electrons by the nucleus of the neighbouring molecule. We know that Vander Waals is always present in every atom or molecule. Hence, the Vander Waals dispersion force is acting in this molecule.
Dipole-dipole interaction forces are generated as the electron cloud is pulled by the more electronegative atom in the molecule. Here, from the periodic table we can say that bromine is more electronegative. Hence, the dipoles are towards the four Bromine atoms.
The shape of the molecule of CBr4 is a tetrahedron. Hence, all four bond lengths and bond angles are the same. Due to this phenomena, even though an individual bond is polar, the vector sum of the dipole of any three bonds will always come opposite to the dipole of the fourth bond.
Hence, the molecule as a whole is non-polar. Hence, the dipole-dipole interaction forces are not present here.
Now, for the Hydrogen bond, the main prerequisite is the presence of Hydrogen, which is not present here. Hence, Hydrogen bond is also not possible.
Hence, only the Vander Waals dispersion forces are acting in the molecule of CBr4
Note :
Vander Waals dispersion forces are stronger in the molecules which possess long chains of elements. The reason is that it is more convenient and easier to displace the electrons as the forces of attraction between electrons and the protons in the nucleus are weaker, which is not the case in smaller or compact molecules.
