Question
Question: What are the formal charges on each atom in sulphite, \( SO_3^{2 - } \) and chlorite, \( ClO_2^ - \)...
What are the formal charges on each atom in sulphite, SO32− and chlorite, ClO2− ions?
Solution
Formal charge is a theoretical value assigned to an atom in a molecule which reflects the equal sharing of electrons in a chemical bond, neglecting the electronegativity difference between the atoms. Sketch the Lewis diagram for the given ions to find the value of formal charge of each atom.
Complete answer:
The formal charge can be assigned to an atom with the help of following formula:
F=V−N−2B−(i)
Where, F is the formal charge on the atom, V is the number of electrons of atom in its ground state, N is the number of lone pair of electrons present on the atom and B is the number of bonding electrons in that atom.
The Lewis structure for sulphite ions is as follows:
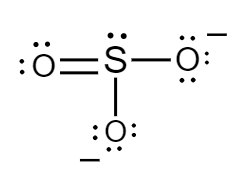
Formal charge for sulphur atom:
Number of electrons of sulphur in its ground state =6
Count of nonbonded electrons present on sulphur atom =2
Number of bonding electrons =8
Substituting values in equation (i) , formal charge of sulphur atom will be as follows:
F=6−2−28
⇒F=0
Formal charge for doubly bonded oxygen atom:
Number of electrons of oxygen in its ground state =6
Count of nonbonded electrons or number of lone pair of electrons present on oxygen atom =4
Number of bonding electrons =4
Substituting values in equation (i) , formal charge of sulphur atom will be as follows:
F=6−4−24
⇒F=0
Formal charge for single bonded oxygen atoms:
Number of electrons of oxygen in its ground state =6
Count of nonbonded electrons or number of lone pair of electrons present on oxygen atom =6
Number of bonding electrons =2
Substituting values in equation (i) , formal charge of sulphur atom will be as follows:
F=6−6−22
⇒F=−1
Hence, in sulphite ions, the formal charge of sulphur atom, doubly bonded oxygen atom and singly bonded oxygen atoms is 0,0 and −1 respectively.
The Lewis structure for chlorite ions is as follows:
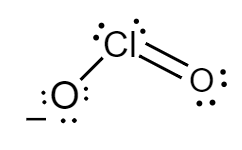
Formal charge for chlorine atom:
Number of electrons of chlorine in its ground state =7
Count of nonbonded electrons present on chlorine atom =4
Number of bonding electrons =6
Substituting values in equation (i) , formal charge of sulphur atom will be as follows:
F=7−4−26
⇒F=0
Formal charge for doubly bonded oxygen atom:
Number of electrons of oxygen in its ground state =6
Count of nonbonded electrons or number of lone pair of electrons present on oxygen atom =4
Number of bonding electrons =4
Substituting values in equation (i) , formal charge of sulphur atom will be as follows:
F=6−4−24
⇒F=0
Formal charge for single bonded oxygen atom:
Number of electrons of oxygen in its ground state =6
Count of nonbonded electrons or number of lone pair of electrons present on oxygen atom =6
Number of bonding electrons =2
Substituting values in equation (i) , formal charge of sulphur atom will be as follows:
F=6−6−22
⇒F=−1
Hence, in chlorite ions, the formal charge of chlorine atom, doubly bonded oxygen atom and singly bonded oxygen atom is 0,0 and −1 respectively.
Note:
Lewis structures in which the formal charges are zero for most atoms in the compound, are more preferably considered than the one with non-zero formal charges. Moreover, the negative formal charge should be present on the most electronegative element in the compound.
