Question
Question: What are the factors which influence the adsorption of a gas on a solid?...
What are the factors which influence the adsorption of a gas on a solid?
Solution
Adsorption is the phenomena by which molecular species accumulate on the surface instead of going deeper into the volume of a solid. It is a surface phenomenon whose rate depends on factors such as temperature, concentration and nature of adsorbate and adsorbent.
Complete step by step answer:
-Adsorption is the loose adherence of gases, liquids, dissolved solids on the surface of another liquid or solid. In adsorption particles so not enter the bulk of another solid or liquid material. The rate of adsorption increases until equilibrium is attained. It is an exothermic process and used in water purifiers, air conditioners, heater etc.
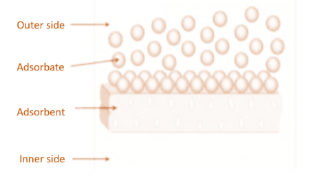
The extent of adsorption of a gas on a solid depends on many factors. These are:
-Nature of adsorbate and adsorbent: Easily liquefiable gases such as ammonia, chlorine, carbon dioxide etc. have higher adsorption properties as compared to elemental gases like nitrogen, oxygen etc. This is due to the fact that easily liquefiable gases have higher intermolecular forces of attraction and thus, they are more strongly adsorbed. An adsorbent is porous and finely powdered like charcoal and adsorbed more as compared to hard and non-porous substances.
-Surface area of the adsorbent: If a solid adsorbent has large surface area, it allows more adsorption to occur. Also, we can say that smaller particle size imparts more surface area.
-Pressure: With an increase in pressure of the adsorbate gas, adsorption increases and this increase is quite significant if the temperature is low. Therefore, we can conclude this as the extent of adsorption is directly proportional to small pressure ranges but attains a limiting value at high pressures when all the adsorption sites are occupied.
-Temperature: We know that adsorption is an exothermic process. As per Le-Chatelier’s principle, a rise in temperature results in reduction of the extent of adsorption. But this is true only in case of physical adsorption because in chemical adsorption, the requirement of high activation energy causes the extent of adsorption to increase initially with rise in temperature but then gradually decreases with rising temperatures.
Hence, these are the factors which influence the adsorption of a gas on a solid.
Note:
We should not get confused between adsorption and absorption. While adsorption is a surface phenomenon, we know that absorption is a bulk process. Adsorption is exothermic but absorption is an endothermic process. Concentration of adsorbed substance changes within the medium but remains constant throughout the medium in absorption.
