Question
Question: What are the electronic configurations of carbon, hydrogen, and oxygen atoms?...
What are the electronic configurations of carbon, hydrogen, and oxygen atoms?
Solution
Hint : Electronic configuration- a way in which electrons are arranged into various orbitals around the nuclei of atoms or distribution of electrons in various orbitals of an atom. Every element has a different electronic configuration.
Complete Step By Step Answer:
There are three rules which are used for the electronic configuration.
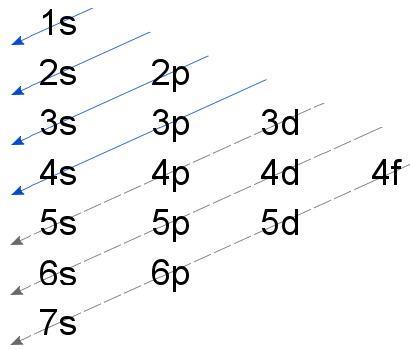
Rule 1. Aufbau principle-It says that the electrons will occupy the lowest energy level orbital that will receive it.
The order in which electrons are filled is as follows: :1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, 8s.
Rule 2. Pauli’s exclusion principle-No two electrons with the same energy characteristics can occupy an orbital at the same time( one electron will have spin up and the other electron will have spin down)
Rule 3. Hund’s rule- While filling the multiple orbitals of the same sublevel (p, d, and f), electrons are first half-filled or singly filled, and then the pairing of electrons takes place.
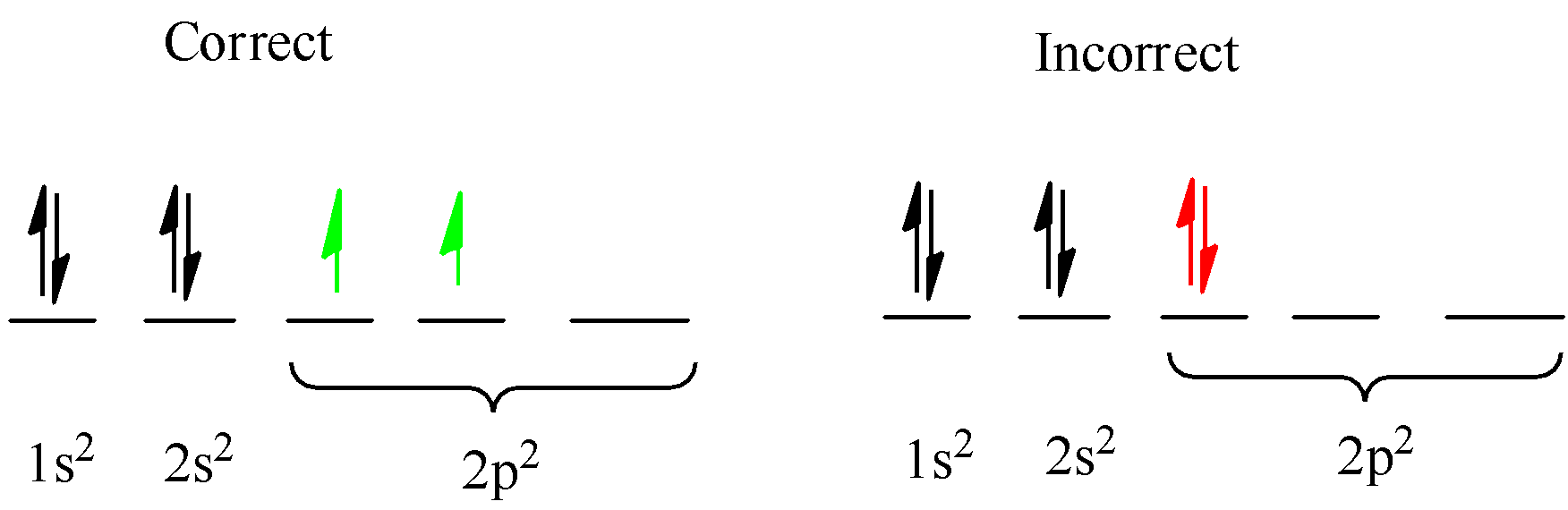
Energy of the orbital increases as follows: n=1<2<3<4<5....∞
And the energy of orbitals increases as: s<p<d<f
The number of electron that each orbital can occupy also differs: s=2,p=6,d=10,f=14 when completely filled and s=1,p=3,d=5,f=7 when half filled
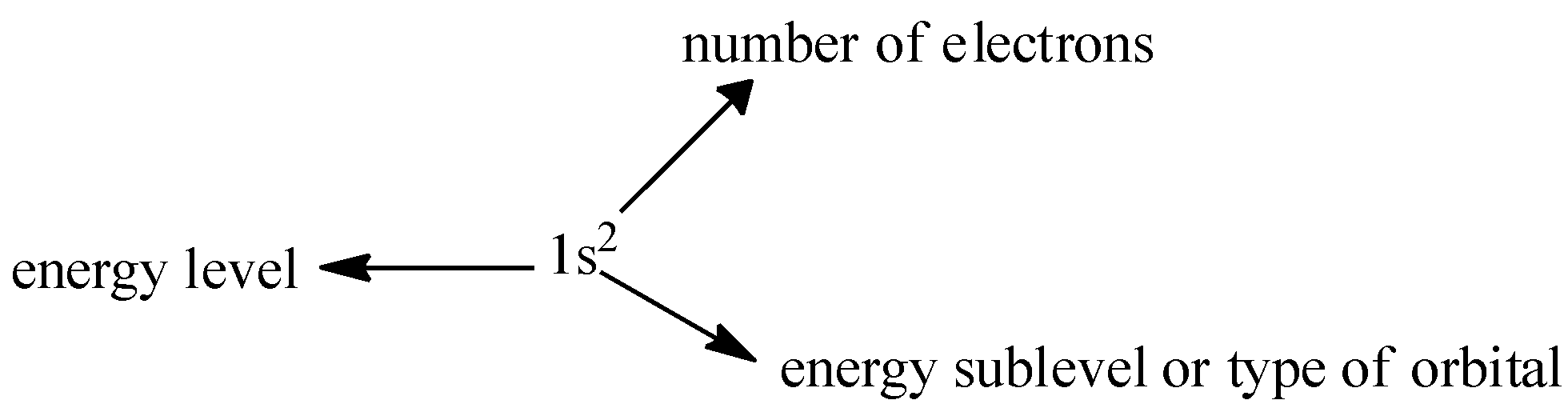
Now let us try to write the electronic configuration starting with carbon.
For writing the configuration we must know the atomic number of that atom and then by following the above rules the electronic configuration of carbon, hydrogen, and oxygen can be written.
Therefore the electronic configurations are:
(Atomic number=6) Carbon = 1s22s22p2 ( 1s orbital can occupy 2 electrons so it is 1s2 so next electrons will go in next orbital 2s if this is completely filled the next orbital will be used and so on)
(Atomic number=1) Hydrogen= 1s1
(Atomic number=8) Oxygen = 1s22s22p4.
Note :
For writing the electronic configuration of any element, one must know the atomic number of elements, or for that periodic table can be used as a reference. Following the rules stated above (Aufbau principle, Pauli’s exclusion principle, and Hund’s rule) are very important for writing the configuration.
