Question
Question: What are the electron deficient and electron rich compounds of hydrogen? Give examples....
What are the electron deficient and electron rich compounds of hydrogen? Give examples.
Solution
Do the general rule of thumb is that there should be 8 electrons present in the valence of any given atom after forming a stable compound. But there are some compounds of hydrogen, which proved to defy this rule.
Complete Step-by-Step Answer:
Before we move forward with the solution of this question, let us first understand some important basic concepts.
Depending on the atomic number of any given element, we can derive its electronic configuration. The electronic configuration of the atom can be used to identify the number of electrons present in the valence shell of the atom. On this basis of this data, we can determine if the given atom would accept electrons or donate electrons to completely fill its valence shell in order to attain maximum possible stability. In almost all the elements, the maximum number of electrons that can be placed in the valence shell of the atom is 8 electrons. But in the valence shell of a hydrogen atom, we can place only 2 electrons. Hence when forming compounds with hydrogen, the hydrogen atom tends to either take 1 electron or lose or electron.
Let us understand few of these cases:
1.There are some compounds like BH3 which do not have 8 electrons in the valence shell of the boron atom, but still form a stable compound. The Lewis structure for this compound can be given as:
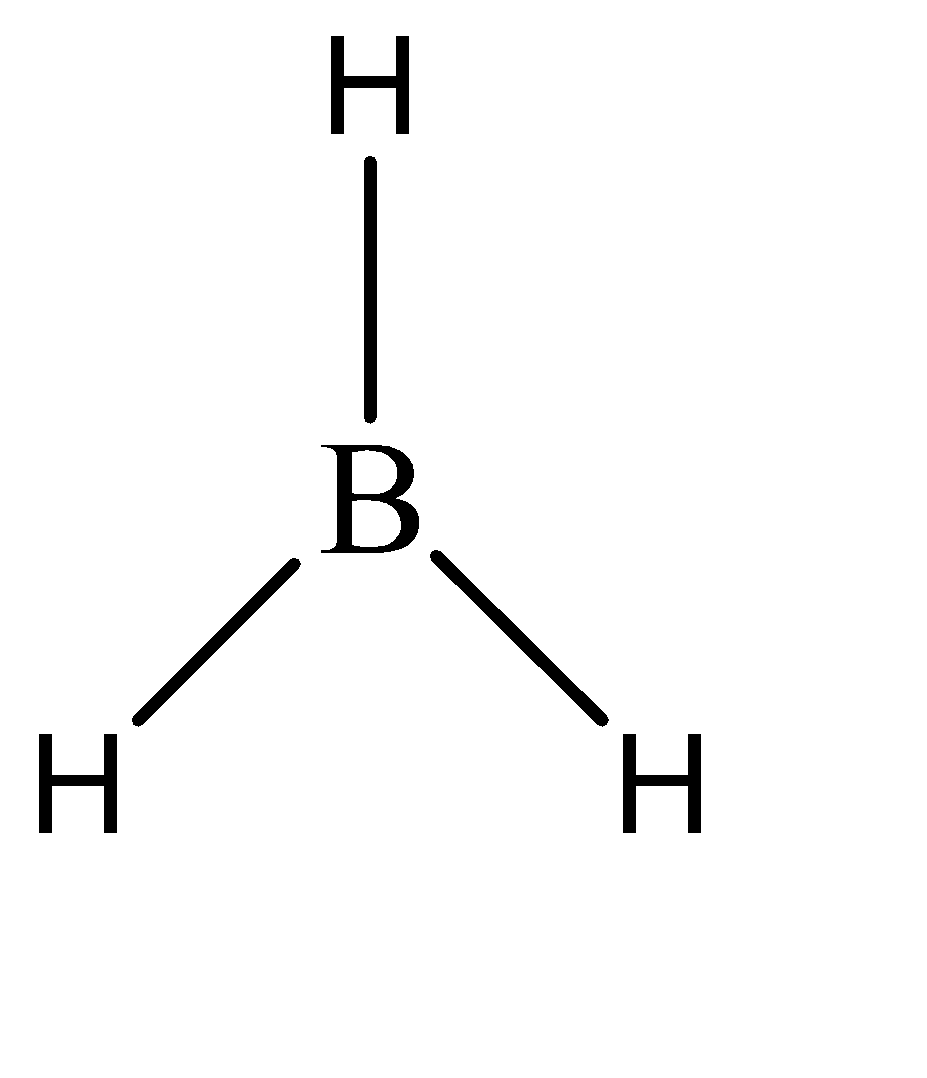
As we can see, there are only three bonds formed by boron. This means that there are only 6 electrons present in the valence shell of boron. Such compounds are called electron deficient compounds.
2.On the other hand, the electron rich compounds of hydrogen can be understood as the compounds which have a greater number of electrons in the central atom, than what is required to form bonds. In simpler terms, these compounds have excess electrons present as lone pairs. Some of the examples are water, ammonia, etc.
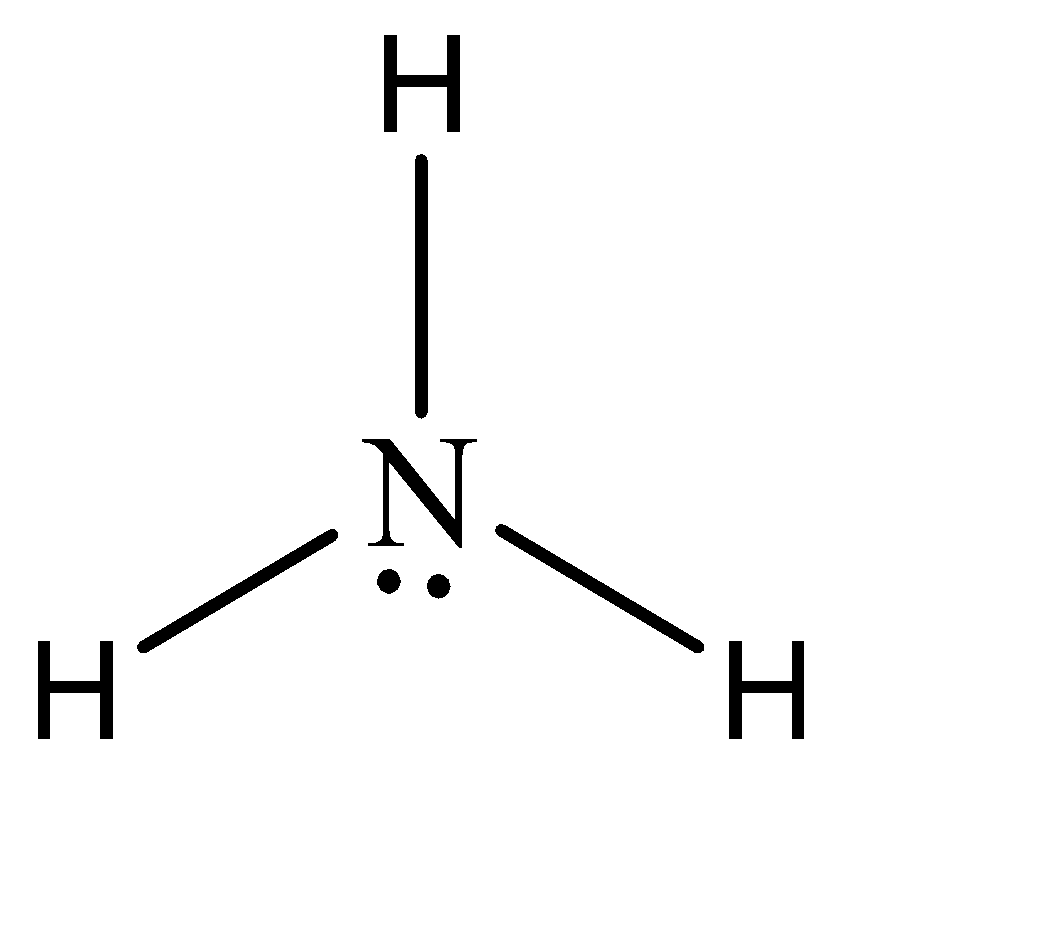
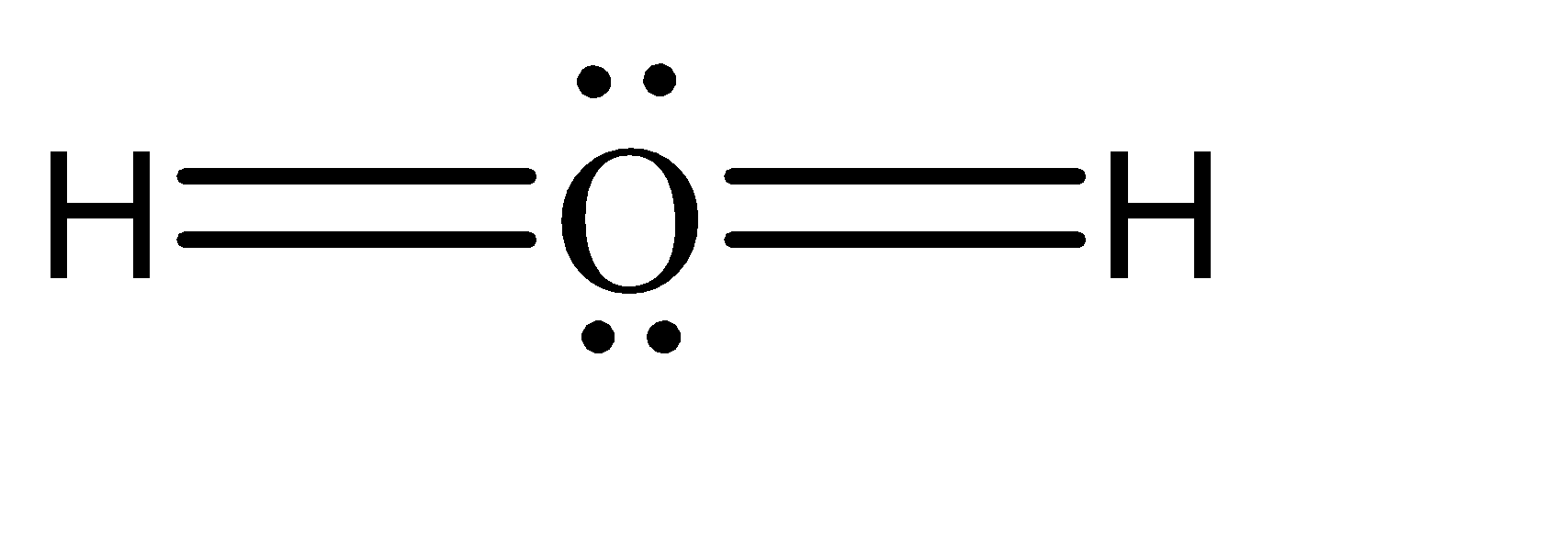
Note: Apart from these two types of compounds, there exists another type called the electron precise compounds. These compounds contain exactly sufficient number of electrons required to form an octet.
