Question
Question: What are the different kinds of f orbitals?...
What are the different kinds of f orbitals?
Solution
Every atom or element in the periodic table contains electrons. All the electrons are going to enter into the fixed orbitals and the electrons continuously move in the stationary orbits. The atomic orbitals are four types and they are s, p d, and f.
Complete answer:
- In the question it is asked to write the different kinds of f-orbitals.
- There are four blocks in the periodic table and they are s, p, d and f blocks.
- The elements are classified into different kinds based on the position of the valence electrons.
- The f-block is positioned at the bottom of the periodic table.
- The f block is going to start with a lanthanum element.
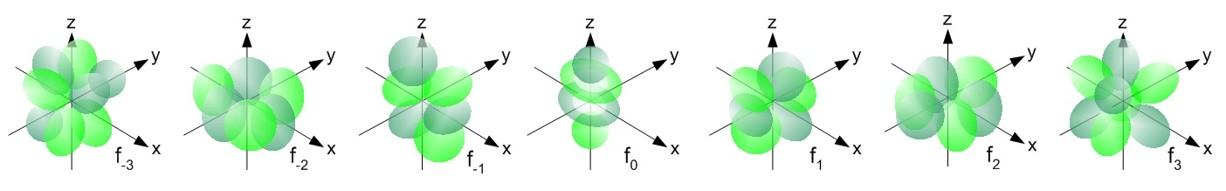
- For the f block elements there 7 f orbitals are present.
- For s orbital there is only an orbital, for p orbital there are three sub-orbitals, for d-orbital there are five sub-orbitals and for f orbital there are 7 sub-orbitals.
- Means f orbital has a capability to accommodate 14 electrons in its orbital.
- The shapes of the f orbitals are as follows, we can see the different shapes of the seven sub-orbitals of the f.
Note:
All atoms in the periodic table do not contain f-orbitals. The elements which are present in the f block only contain f orbitals and they can accommodate fourteen electrons in their sub-orbitals. The f orbitals are very complex.
