Question
Question: What are the crystal field splitting energy and the spin only moment in Bohr magneton for the comple...
What are the crystal field splitting energy and the spin only moment in Bohr magneton for the complex K3[Fe(CN)6]?
Solution
The crystal field theory describes the conversion of five d− orbitals of metal ions which are already in generated form into the different sets of orbitals which are having variation in energies in the presence of electric field applied by the ligands.
Complete answer:
In the octahedral complexes, where central metal has 6 ligands, all the 5 orbitals of d− orbitals do not have the same energy level. Two of these orbitals namely dz2,dx2−y2 possess high negative charge and have higher energy levels known as (eg).
Remaining three orbital dxy,dyz,dzx have less negative energy and experience low repulsion energy from the ligands and have a low energy state known as (t2g).
Electronic configuration of (Fe) is 1s22s22p63s23d64s2. After removal of three electron iron atom get converted into its ionic form (Fe+3) and electronic configuration will become 1s22s22p63s23d54s0.
As we see d− orbitals of (Fe+3) have a total of 5 electrons they get arranged into orbitals depending upon the energy. Cyanide causes splitting of crystal field upto large extent and hence it comes under the category of strong field ligand.
As the complex contains cyanide ion which acts as a strong ligand it promotes pairing of electrons in d− orbitals of (Fe+3).
Hence, in starting three electrons fill into dxy,dyz,dzx and in the second round remaining two electrons also fill into these orbitals due to cyanide ligand.
Therefore, all the electrons of (Fe+3) are present at (t2g) energy state.
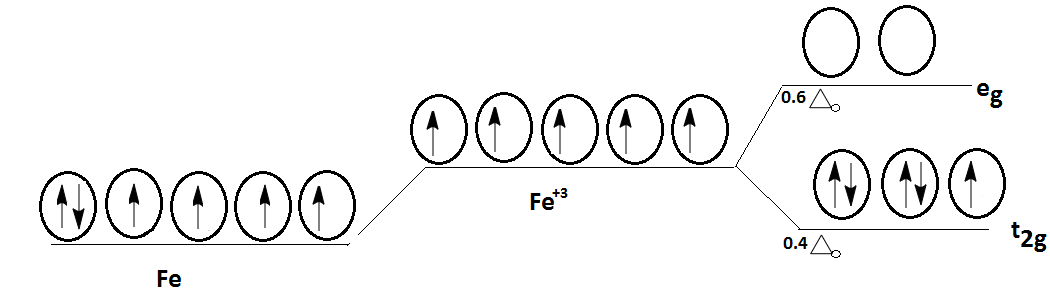
Due to pairing of electrons in d− orbitals, this complex has low spin.
Note:
Weak ligands do not promote pairing and therefore they form high spin complexes. Other examples of strong field ligands include (NO2−,CO). The ligands which cause splitting of crystal field only upto small extent are known as weak field ligands like (I−,Cl−).
