Question
Question: What are the correct oxidations state, coordination number, configurations, magnetic character and m...
What are the correct oxidations state, coordination number, configurations, magnetic character and magnetic moment of K4[Mn(CN)6]?
O.S. C.N. Configu- Magnetic Magnetic
-ration Character moment
A
+6 6 t2g5 Diamagnetic 0
B
+4 6 t2g4eg1 Paramagnetic 1.732B.M
C
+2 6 t2g5 Paramagnetic 1.732B.M
D
+4 6 t2g3eg2 Diamagnetic 0
Answer
+2 6 t2g5 Paramagnetic 1.732B.M
Explanation
Solution
O.S. +2, C.N. = 6, configurations =t25g
Mn2+=4s03d5
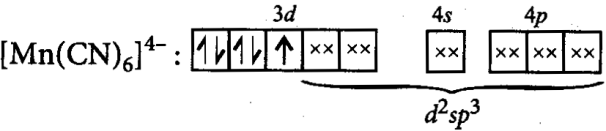
It has one unpaired electron and hence it is paramagnetic in nature.
μ=1(1+2)=3=1.732B.M.
