Question
Question: What are the chiral centers in sucralose?...
What are the chiral centers in sucralose?
Solution
Sucralose is a disaccharide derivative consisting of two simple carbohydrate units linked by a glycosidic bond. The molecular formula of sucralose is C12H19Cl3O8 . The chiral centre means the carbon with four different groups. They are a total of 9 chiral centres in sucralose.
Complete answer:
Sucralose is a disaccharide derivative, disaccharide means upon hydrolysis, it will give two simple molecules of monosaccharide units like glucose. Sucrose is also an example of disaccharide. The molecular formula of sucralose is C12H19Cl3O8 . whereas the molecular formula of sucrose is C11H22O11 .
Chiral centre means the carbon attached to four different groups. By looking at the structure of sucralose, the chiral centres can be known.
The structure of sucralose is
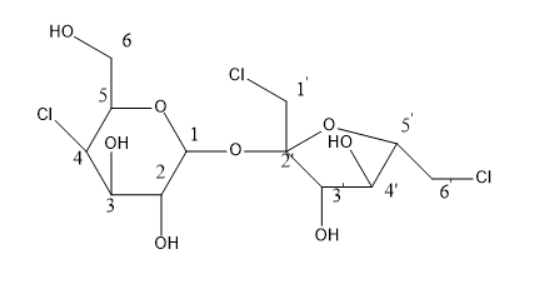
In the above structure, there is a total of 12 carbons. Out of 12 carbons, 1′ has two hydrogen atoms, 6′ has two hydrogen atoms and 6 carbon has also two hydrogen atoms. Thus, these three carbon centres are not chiral. Whereas the remaining carbons are all chiral centres.
Thus, the chiral centres are 1,2,3,4,5,2′,3′,4′, and 5′ . As these all carbons have four different groups attached with them and are known as chiral centres.
The chiral centres in sucralose are 9 and these are 1,2,3,4,5,2′,3′,4′, and 5′ position carbon atoms.
Note:
Chiral centres were important to determine the optical activity and also in the determination of configuration of alkanes containing four different groups which is known as chirality. The Entgegen (E) and Zusammen (Z) configuration can be written to the alkanes with chirality.
