Question
Question: What are the bond angles in the central atoms of the following: NSF, \(O{{F}_{2}}\), and \(IB{{r}_{2...
What are the bond angles in the central atoms of the following: NSF, OF2, and IBr2− ?
Solution
Bond angle of any atom in a molecule is affected by the lone pairs of electrons as well as the bonds that the atoms form in that molecule. Multiple bonds are shorter in length.
Complete answer:
Bond angle is the measure of the angle between adjacent atoms in a molecule. The bond angle decides the geometry on any molecule. These angles are affected by the lone pair of electrons on the atom.
For determining the bond angles, we look at the Lewis structures of the given compounds. The bond angles are expressed as,
For NSF,
The Lewis structure of NSF is, N¨≡S¨−⋅⋅F¨: , this shows that there is 1 lone pair of electron each on N and S, while 3 lone pair of electrons on F. This makes the type of the molecule to be AX2E type, so the molecular shape becomes bent, as
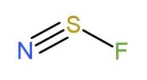
Due to the bent shape, the bond angle of the central atom is 120∘, but due to repulsion of lone pair it decreases to nearly 117∘.
For OF2,
The Lewis structure is, :⋅⋅F¨−⋅⋅O¨−⋅⋅F¨:, this shows 3 lone pair of electrons on both the F atoms, and 2 lone pairs on O. This makes the type of the molecule to be AX2E2, so the molecular shape becomes bent, as
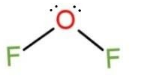
Due to bent shape, the bond angle of the central atom is 109.5∘, but it decreases to nearly 103∘ due to repulsion in electrons.
For IBr2−,
The Lewis structure is, [:⋅⋅B¨r−⋅⋅−⋅⋅B¨r:]− , this shows, 3 lone pair of electrons on both the Br, and 3 lone pair on I. This makes the molecule to be of AX2E3 type, with trigonal bi-pyramidal geometry, as
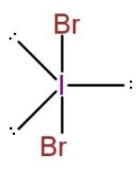
The bond pairs are on axial positions, while lone pairs on equatorial position, this makes the bond angle of the central atom to be 180∘.
Hence, the bond angles of central atoms of molecules NSF, OF2, and IBr2− are near 117∘, near 109.5∘, and 180∘respectively.
Note: The types of molecules and their geometry according to the number of bond pairs and lone pairs is well explained in the VSEPR, valence shell electron pair repulsion theory.
