Question
Question: What are the bond angles in\[{{H}_{3}}O\]?...
What are the bond angles inH3O?
Solution
Bond angles are the angles between the adjacent atoms in the molecule. The bond angle in any molecule varies with the presence of the bond pair of electrons and the lone pair of electrons according to the VSEPR theory. The H3O molecule is present in the form of cation H3O+called as hydronium ion whose geometry is pyramidal.
Complete answer:
The shapes of molecules affect the bond angles between them, which are the angles between adjacent atoms. These shapes are identified using VSEPR theory that stands for valence shell electron pair repulsion theory. This theory suggests that a shape of a molecule depends on the valence pair of electrons in the atoms of that molecule. These valence electrons are distributed in the form of bond pairs and lone pairs. The interactions of these pairs are in the order, lone pair – lone pair > lone pair – bond pair > bond pair – bond pair. This implies that lone pair-lone pair interaction is more due to which the shape of the molecule distorts and the bond angle changes.
We have been given H3O molecule that is present in the form ofH3O+ called hydronium ion. This ion consists of an irregular geometry. The total valence electrons in the molecule are 3 in hydrogen and 5 in oxygen cation that makes 8. They are distributed as lone and bond pairs, among which 6 electrons form 3 bond pairs and the remaining 2 electrons consist of lone pairs on oxygen. This makes the hybrid state of the molecule to be sp3 and the shape pyramidal. Due to pyramidal shape all the bond angles become 107∘. The molecule is,
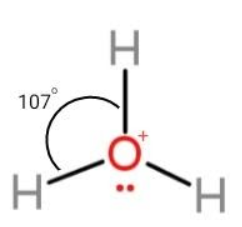
Hence, the bond angles inH3O+ molecules are 107∘.
Note:
The hybrid state according to the VSEPR can be determined by a formula through which the shapes of the molecules can be identified. The formula is X=SA+21(G−V±E), whereSA is number of surrounding atoms, X is hybrid state, G is the valence electrons in central atom, V is valence of surrounding atoms, E is charge (added when negative, subtracted when positive). For, H3O+it gives X = 4 that shows, the sp3pyramidal shape, same as NH3.
