Question
Question: What are the blocks, groups and periods for the following Electronic Configuration periodic table? I...
What are the blocks, groups and periods for the following Electronic Configuration periodic table? Is 1s22s22p63s13p33d
(a) p-block ,VI-A , 3rd period
(b) p-block ,VII-A , 3rd period
(c) p-block ,V-A , 3rd period
(d) p-block ,VI-A , 4rd period
Solution
The shell in which the last electron enters tells us about the block to which the element belongs and the value of principal quantum number tells us about the period and for s-block elements the group number is equal to the number of the valence electrons and for p-block elements the group number =10+ number of electrons in the valence shell. Now answer the statement.
Complete step by step answer:
-In the modern periodic table, the physical and chemical properties of the elements are the periodic functions of their atomic weights and the long period table has been divided into the periods (horizontal rows) and groups (vertical columns). In terms of the electron configuration, a group consists of a series of elements which have the same outermost electronic configurations but they have different total numbers of electrons. In the periodic table, there are a total of 18 groups and 7 periods. The elements belonging to the groups 1and2 belong to s-block elements and have the general electronic configuration as ns1−2 and the elements belonging to the groups 13and 18 belong to p-block elements and have the general electronic configuration as ns2np1−6. From the statement, we can see that the outermost configuration is the same as the p- block elements and the last electron also enters the p-orbital. So, thus, the element is a p- block element. For p-block elements, to find the group the formula is applied is as: Group number= 10+ the total number of valence electrons
-As the number of electrons in the valence is 4. Then the Group number= 10+4=14 or V-A.
We can know about the period from the principal quantum number. The principal quantum number tells us about the period or the shell to which an electron belongs. The highest value of the principal quantum represents the period of the element. In the given electronic configuration, the highest value of principal quantum number is 3. So, it belongs to the third period. Hence, the element with the electronic configuration -1s22s22p63s13p33d belongs to 3rd period, V-A group and is a p-block element.
So, option (c) is correct.
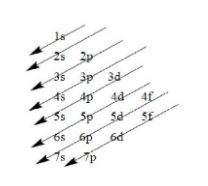
Note: When we write the electronic configuration of any element, the electrons are filled according to the Aufbau principle i.e. the electrons are first filled in the orbitals having lower energy followed by orbitals having higher energy as indicated by the arrows.