Question
Question: What are the 9 isomers of Heptane?...
What are the 9 isomers of Heptane?
Solution
Isomers are those compounds which have the same molecular formula but have different spatial arrangement. Heptane is an alkane having seven carbon atoms. Hence, the chemical formula for heptane is C7H16.
Complete answer:
The chemical formula for heptane is C7H16. The structure of first isomer named as n-heptane is given as follows:
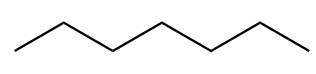
The structure of second isomer named as 2-Methylhexane is given as follows:
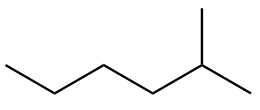
The structure of third isomer named as 3-Methylhexane is given as follows:
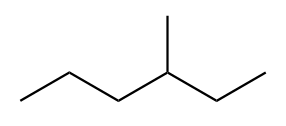
The structure of fourth isomer named as 2,2-Dimethylpentane is given as follows:
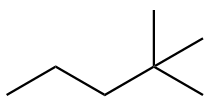
The structure of fifth isomer named as 2,3-Dimethylpentane is given as follows:
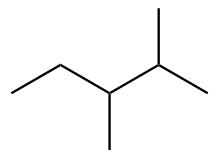
The structure of sixth isomer named as 2,4-Dimethylpentane is given as follows:
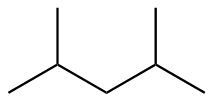
The structure of seventh isomer named as 3,3-Dimethylpentane is given as follows:
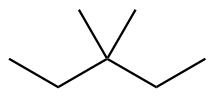
The structure of eighth isomer named as 3-Ethylpentane is given as follows:
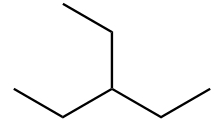
The structure of ninth isomer named as 2,2,3-Trimethylbutane is given as follows:
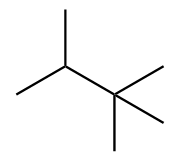
From the above structures, we can see that all structures differ in structural arrangement in space and have the same molecular formula.
Therefore, the 9 isomers of heptane are n-heptane, 2-Methylhexane, 3-Methylhexane, 2,2-Dimethylpentane, 2,3-Dimethylpentane, 2,4-Dimethylpentane, 3,3-Dimethylpentane, 3-Ethylpentane and 2,2,3-Trimethylbutane.
Note:
It is important to note that isomers have the same chemical formula but have different structures. The 9 isomers of heptane differ in the number of carbon atoms in the parent chain. The IUPAC name of these isomers are n-heptane, 2-Methylhexane, 3-Methylhexane, 2,2-Dimethylpentane, 2,3-Dimethylpentane, 2,4-Dimethylpentane, 3,3-Dimethylpentane, 3-Ethylpentane and 2,2,3-Trimethylbutane.
