Question
Question: What are strong and weak acids? In the following list of acids, separate strong acids from weak acid...
What are strong and weak acids? In the following list of acids, separate strong acids from weak acids. Hydrochloric acid, citric acid, acetic acid, nitric acid, formic acid, sulphuric acid.
Solution
We know that there are two types of substance, i.e., acids and base, these are classified on the basis of ions produced by their solution, i.e., hydrogen ions and hydroxyl ions. Extending the ions produced tells whether the compound is strong or weak.
Complete step-by-step answer: We know that there are two types of substance, i.e., acids and base, these are classified on the basis of ions produced by their solution. The acids produce hydrogen ions whereas the base produces hydroxyl ions.
We can decide the compound is a strong acid or weak acid on the basis of the extent of hydrogen ions produced in the solution, so the acids that get fully ionized in the solution then it is a strong acid and the acids get partially ionized in the solution then it is a weak acid.
Hydrochloric acid having the formula HCl is a strong acid because when it is dissolved in water it completely dissociates into H+ and Cl− ions.
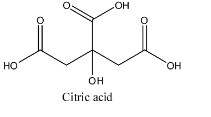
Citric acid having formula C6H8O7 is a weak acid because when it is dissolved in water it partially dissociates into ions and remains in equilibrium. The structure is given below:
Acetic acid having formula CH3COOH is a weak acid because when it is dissolved in water it partially dissociates into ions and remains in equilibrium.
Nitric acid having formula HNO3 is a strong acid because when it is dissolved in water it completely dissociates into H+ and NO3− ions.
Formic acid having the formula HCOOH is a weak acid because when it is dissolved in water it partially dissociates into ions and remains in equilibrium.
Sulphuric acid having formula H2SO4 is a strong acid because when it is dissolved in water it completely dissociates into H+ and SO42− ions.
Note: In the same way we can classify the bases as a strong base and weak base, compounds that ionize fully are called strong bases, and compounds that partially ionize are called weak bases.
