Question
Question: What are organometallic compounds? Write an example....
What are organometallic compounds? Write an example.
Solution
We had studied organic and inorganic compounds. We know that organic compounds essentially have carbon whereas the inorganic generally do not have carbon. So, organometallic is also the category of compounds based upon the atoms present in it. The name organometallic itself indicates its meaning. When we break this word into two, we will get organic and metallic.
Complete step-by-step answer:
The organometallic compounds contain organic structure and metal. We had study transition metal compounds in coordination chemistry having ligand as −NH2, −PR3 are inorganic compounds. When we replace the inorganic ligand with organic compounds the compounds contain metal and organic compounds as ligands, so the formed compounds are known as organometallic compounds.
Organometallic compounds necessary have at least one metal-carbon bond.
The example of organometallic compounds are given as follows:
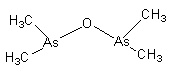
The name of the above given compound is dimethylarsinic anhydride. It is also known as Cacodyl oxide. It is the first organometallic compound of the p-block element.
Organometallic compounds are formed by s-block, p-block elements, transition metals, lanthanides and actinides.
NaC5H5− is the example of s-block organometallic compounds. Ni(CO)4 is the example of transition metal organometallic compound. U(C8H8)2 is known as uranocne is an example of lanthanide organometallic compound
Note: The bonding in organometallic compounds is ionic and covalent. Some compounds do not have metal-carbon bonds even though they are considered as organometallic compounds. The example of Wilkinson catalyst whose formula is RhCl(PPh3)3. It is used for hydrogenation of alkene. Carbon monoxide is an inorganic compound but the metal carbonyls are considered as organometallic compounds.
