Question
Question: What are ‘fuel cells’? Write cathode and anode reaction in a fuel cell....
What are ‘fuel cells’? Write cathode and anode reaction in a fuel cell.
Solution
Fuel cell, as the name suggests, is the cell which uses fuel to generate energy. It is an electrochemical cell, consisting of two metal rods, called electrodes, dipped in an electrolyte solution. At the cathode, reduction reaction occurs, and at the anode, oxidation reaction occurs.
Complete Solution :
Fuel cells are a kind of voltaic cells which convert chemical energy to electrical energy. Unlike other voltaic cells, it can be run indefinitely provided with continuous supply of fuel. The fuel can be H2, CO, CH4, etc. Most common fuel cells use H2 as a fuel.
Let us study the hydrogen-oxygen fuel cell in detail to know the reaction taking place at anode and cathode. The main chemical reaction responsible for producing electrical energy is combustion of hydrogen gas. For that we shall refer to the typical diagram of fuel cells given below:
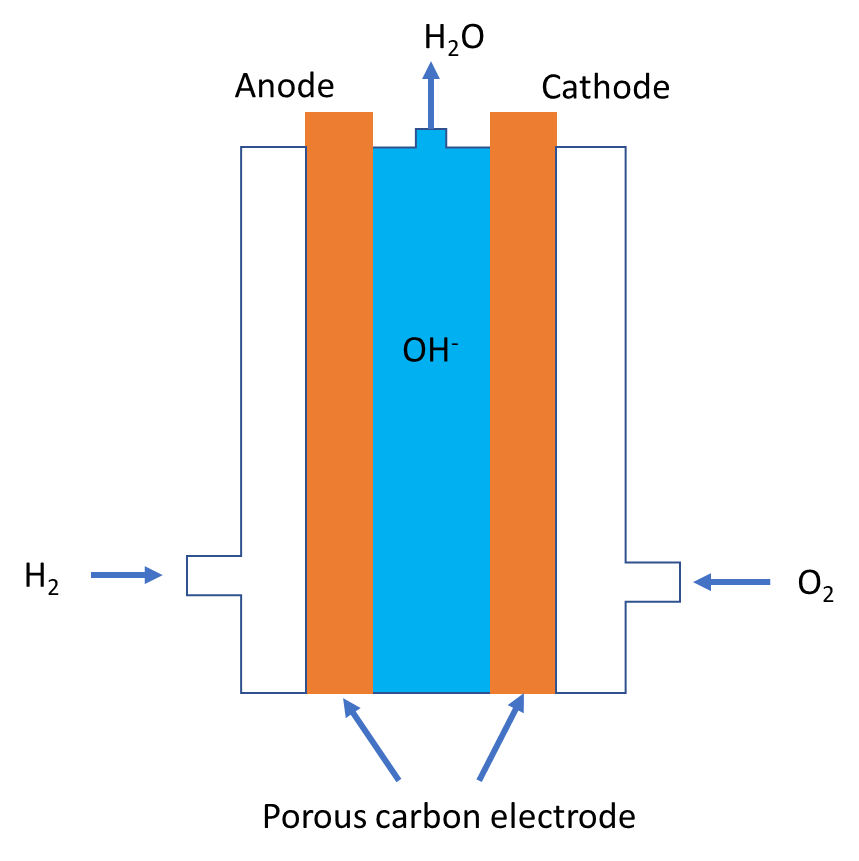
- It contains two porous carbon electrodes, which can pass fuel gas and oxygen through it and conduct electricity. Sodium hydroxide or potassium hydroxide is used as electrolyte. Hydrogen gas is supplied at anode and oxygen is supplied at cathode. The net chemical reaction of the fuel cell is given below.
