Question
Question: What are free radicals? Draw the structure of various types of free radicals and arrange them in inc...
What are free radicals? Draw the structure of various types of free radicals and arrange them in increasing order of their stability.
Solution
We have heard about free radicals several times. They have a major role in the atmospheric industry, combustion, polymerization, biochemistry etc. They are also useful in several biological processes. They have a role in biological metabolism.
Complete step by step answer:
As it is familiar that the species of molecules having an unpaired electron are termed as free radicals. They are represented as X∙ and are highly reactive species. Photo irradiation is a common method to produce free radicals. Light or heat in the form of energy is needed for the production of free radicals. Free radicals are classified into σ and π radicals. The unpaired electron is in σ orbitals in σ radicals. E.g. phenyl radical, vinyl radical etc. While the unpaired electron is in π orbitals in π radicals. E.g. t-butyl radical. π radicals are generally stabilized by hyperconjugation effect or resonance effect. While there is no such stabilizing effect in σ radicals.
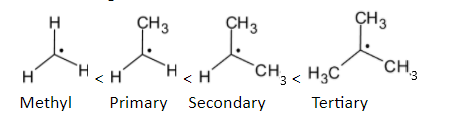
In the case of alkyl groups, when the number of alkyl groups on the carbon having free radicals is increased, the stability is increased.
The order of stability is given below:
Methyl radicalThe electron-deficient radical can be delocalized on the multiple bonds with carbon atoms. Stability is increased when a partially filled p orbital is participated in resonance with the adjacent p orbitals. The structure is given below:
When we compare the stability of phenyl and vinyl radicals, the order is given below:
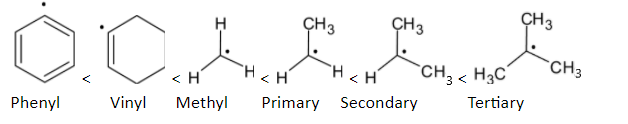
Note: The stability of free radicals depends upon three factors:
Increase in the number of alkyl groups on the carbon atom which has free radical.
Delocalization or resonance
Geometry of free radicals
