Question
Question: What are examples of geometric isomers?...
What are examples of geometric isomers?
Solution
Isomers are the compounds having the same molecular formula but they differ in structural formulas. There are various types of isomers like, position isomers, functional isomers, geometrical isomers, stereoisomers, etc. They all only differ in arrangement of the atoms in the structures.
Complete answer:
Isomerism is the property of any compound to have the same molecular formula but different structural formulas. The compounds having hindered rotation in space around a specific carbon – carbon double bond or single bond, that is the alteration in the spatial arrangement of molecules around the carbon atoms result in isomerism called geometrical isomerism and the isomers are called geometric isomers. There are 2 types of geometric isomers, ‘cis’ and ‘trans’.
-cis isomers: when similar groups are present on the same side of the double bonds, then they are termed as cis.
- trans isomers: when similar groups are present on the opposite sides of the double bonds then they are called trans isomers.
Some examples of cis and trans isomers are as follows:
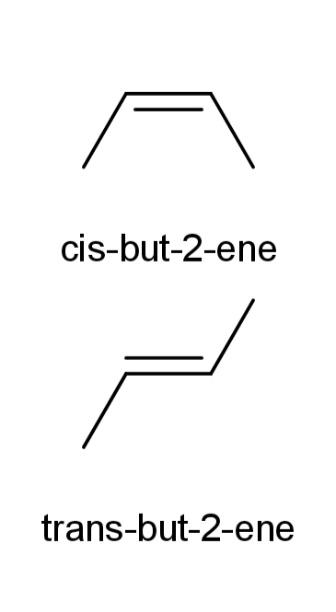
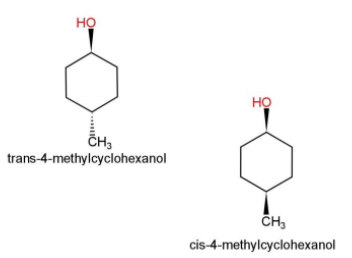
Cis, shows the alkyl groups or the functional groups on the same side of the carbon atom, while trans shows the alkyl and functional groups in both the cases on different sides of the carbon atoms.
Hence, cis and trans isomers are examples of geometric isomers.
Note:
These cis and trans isomers have same chemical formula but they differ in their physical properties. Trans isomers have high melting points due to symmetry than cis isomers. While, cis isomers have high boiling point than trans, due to the presence of polarity due to dipole moment.
