Question
Question: What are cationic detergents? Give an example....
What are cationic detergents? Give an example.
Solution
We know that detergents are agents of cleaning that are made from resources which are synthetically available like petroleum fraction, coal (or) hydrocarbon. It is a surfactant that decreases the water’s surface tension to make it very difficult to bind with water and more readily contact with oil (or) grease. Cationic detergents and anionic detergents are two types of detergents.
Complete step by step answer:
We can say that detergents are sodium salts of sulfonic acids. These were progressed during the 2second world water because of the shortage of fats of animals and oils of vegetables.
Cationic detergents are those that give electrically positive ions in solution. The quaternary salts of ammonium of amines with acetate, chlorides or bromides as anions are cationic detergents. They contain cations at the soluble terminal of the chain. These are long-chain hydrocarbons containing a positive charge on nitrogen atoms. The long-chain cation accounts for their surface-active properties. An example of cationic detergent is cetyltrimethylammonium bromide. This compound is used in hair conditioners.
The structure of cetyltrimethylammonium bromide could be drawn as,
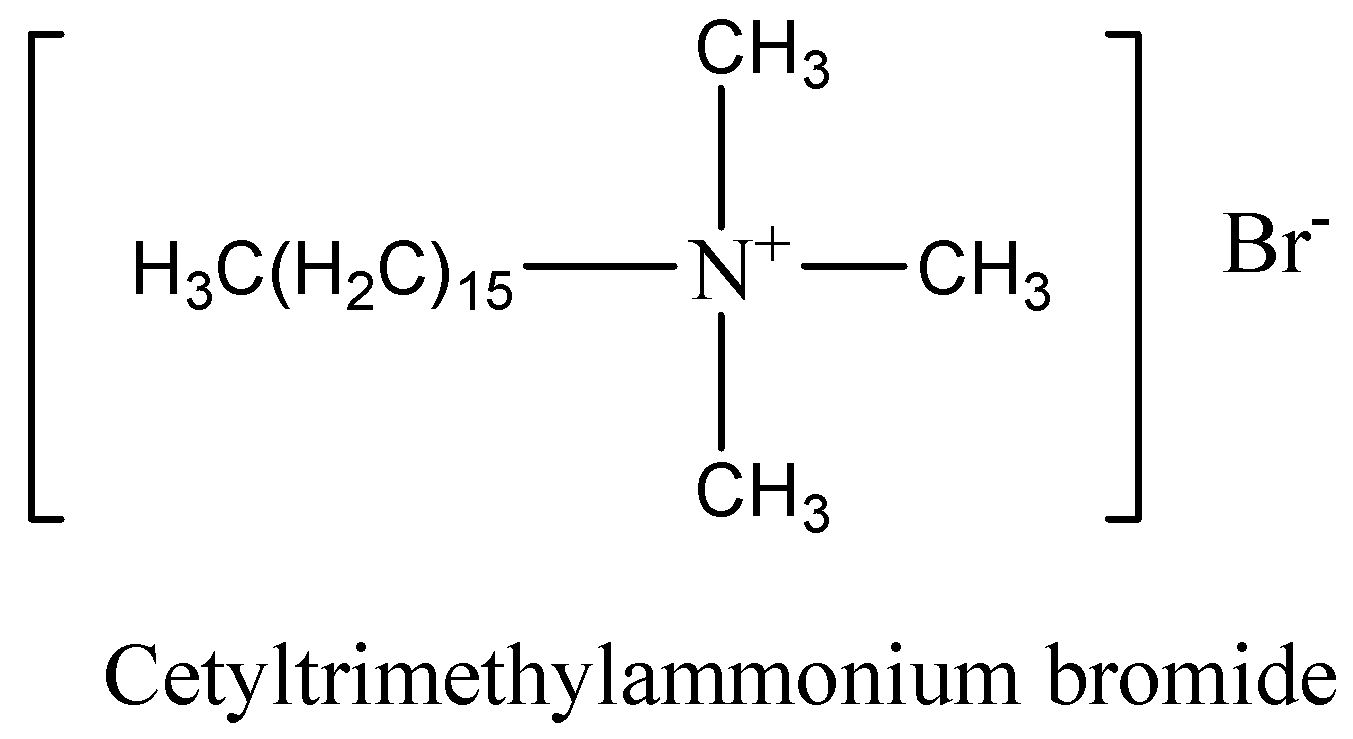
We have to know that the cationic-active agents are utilized as emulsifying agents for asphalt in the roads surfacing. These agents absorb powerfully on minerals, mainly on silicates, and hence make a strong bond between the asphalt and the aggregate. Cationic detergents also hold excellent germicidal properties and are used in surgery in dilute form.
Note: We must remember that the cationic surfactant in solution has the head positively charged. In fabric softeners and in detergents, cationic surfactants give softness. Esterquat is an example of cationic surfactant. In laundry detergents, cationic surfactants enhance the packing of molecules of anionic surfactant at the interface of water/stain.
