Question
Question: What are A and B in the following reaction? 
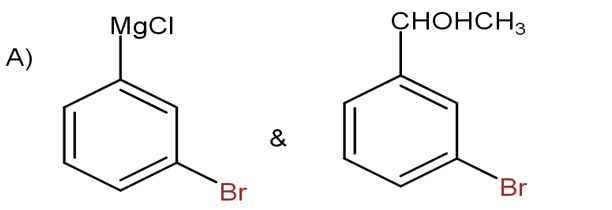
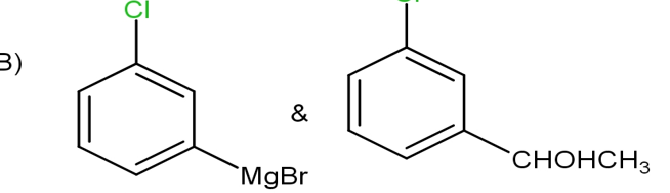
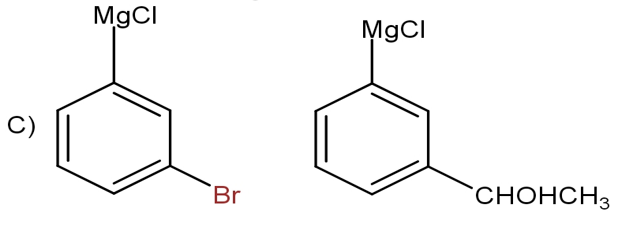
D) None of these
Solution
When the cycloalkyl halides with different halogens treated with magnesium then the magnesium attacks on higher electron density halogen only, leads to the formation of Grignard reagent. The Grignard reagent when treated with aldehyde forms hydroxy compounds.
Complete answer:
Periodic table is the representation of elements arranged in the order of increasing atomic numbers.
These elements are arranged in vertical columns and horizontal rows.
The vertical columns are called groups and the horizontal rows are called periods.
There are 18 groups and 7 periods in the periodic table.
The 17th group elements are called halogens.
Chlorine and bromine are the halogens.
Halogens are more electronegative than the other elements in the periodic table.
Given compound is 3 -bromo chlorobenzene.
When it is treated with Magnesium in presence of tetrahydrofuran (THF) the magnesium attacks on the chlorine atom only, as the chlorine is more electronegative means it has more electron density then bromine atom.
Thus, the compound formed is
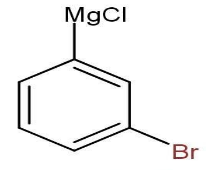
When this newly formed compound is treated with acetaldehyde in presence of ammonium chloride, the magnesium chloride is replaced by aldehyde and forms hydroxyl group.
Thus, the compound formed is
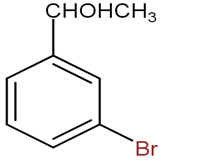
The option B is not correct, as the magnesium attacks on the bromine atom which is not true.
In the Option C, the aldehydic carbon is replaced by bromine atom which is not true.
Hence, the remaining options are not true.
Thus, Option A is the correct one.
Note:
The electron density of halogens must be identified properly. Electron density is more for the electronegative elements. Electronegativity generally decreases down the group. As magnesium is electropositive it attacks on the halogen with high electron density only.
