Question
Question: What amount of bromine will be required to convert \(2g\) of phenol into \(2,4,6 - \) tribromophenol...
What amount of bromine will be required to convert 2g of phenol into 2,4,6− tribromophenol?
A. 4.0
B. 6.0
C. 10.2
D. 20.4
Solution
The stoichiometry of the reaction is the deciding factor of about how much weight of any reactant do you require yielding the product. The reactants react with each other in a simple integral ratio and this helps in defining the physical analysis of the reacting materials.
Complete step by step answer:
Phenol is an aromatic organic compound with the molecular formula C6H5OH . It is a white crystalline volatile solid. The molecule consists of a phenyl group bonded to a hydroxyl group. Being mildly acidic, it requires careful handling because it can cause chemical burns on the skin and can even cause irradiation of eyes.
When phenol reacts with excess of bromine, it produces 2,4,6− tribromophenol. The reaction is as follows:
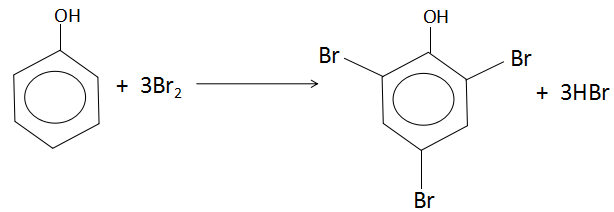
As per the reaction, one mole of phenol reacts with three moles of bromine. One mole of any substance is equal to the molar mass of that substance. Let us calculate the molar masses of the reactants.
Phenol = C6H5OH=(6×12)+(6×1)+(1×16)=94g
Bromine = Br2=160g
Hence, we can clearly see from the reaction that 1mole of phenol reacts with 3moles of bromine. This means:
(1×94)g of phenol reacts with = (3×160)g of bromine
1g of phenol will react with = 943×160g of bromine
Hence, 2g of phenol will react with = 2×943×160=10.2g of bromine
Thus, the correct option is C. 10.2 .
Note:
The water that we see in hydrated salt is called water of crystallisation. This even imparts
colour to salts. We should not overheat the salt, so much that the salt itself will start dissociation.
But we need only loss of water, so heating has to be done under proper care.
