Question
Question: Westron has the formula:- (a)- \(C{{F}_{2}}C{{l}_{2}}\) (b)- \(CHC{{l}_{3}}\) (c)- \({{C}_{2...
Westron has the formula:-
(a)- CF2Cl2
(b)- CHCl3
(c)- C2H2Cl4
(d)- CHF3
Solution
Westron is a chemical compound in which there is a total of 8 elements. There is an element in the Westron that belongs to group 17 and in period three of the periodic table that means the atomic number of the element is 17.
Complete step-by-step answer: Westron is an organic compound in which there is a total of 8 elements. There are three elements that in different ratios form the Westron compound. There are carbon atoms, hydrogen atoms, and chlorine atoms are present. There are 2 atoms of carbon element, 2 atoms of hydrogen element, and 4 atoms of chlorine element. So, the formula will be C2H2Cl4. Its IUPAC name is 1, 1, 2, 2-Tetrachloroethane. The structure of Westron is given below:
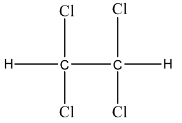
There are other names of Westron like TeCA, s-Tetrachloroethane, Acetylene tetrachloride, etc.
The molecular mass of Westron is 167.848 g/mol. The color of Westron lies between colorless to pale yellow and its physical state is the liquid state. Since this compound has chlorine atoms, therefore, its odor is pungent and chloroform-like.
The density of Westron is 1.59 g/cm3.
The melting point of Westron is −44∘C or 229 K and the boiling point is 146.5∘Cor 419.6 K.
The solubility of Westron in water is equal to 1g per 350mL.
In industries, the use of Westron is in the production of trichloroethylene, tetrachloroethylene, and 1, 2,-dichloroethylene.
Therefore, the correct answer is option (c)- C2H2Cl4.
Note: The Westron solvent is a very hazardous solvent that can cause many types of disease like Jaundice, enlarges the liver, headaches, tremors, dizziness, drowsiness, etc. This solvent is even banned in the United States.
