Question
Question: Waves inside a gas are \(\left( a \right)\) Longitudinal \(\left( b \right)\) Transverse \(\le...
Waves inside a gas are
(a) Longitudinal
(b) Transverse
(c) Partly longitudinal, partly transverse
(d) None of these
Solution
Hint – In this question use the basic concept that in total there are 3 categories of waves in the real world that are the longitudinal waves, transverse wave and the standing wave. Since in gases there is no force present to cause perpendicular motion, thus waves inside a gas surely not traverse. Now only two options left that are standing or longitudinal, use the basic definitions to reach the right answer.
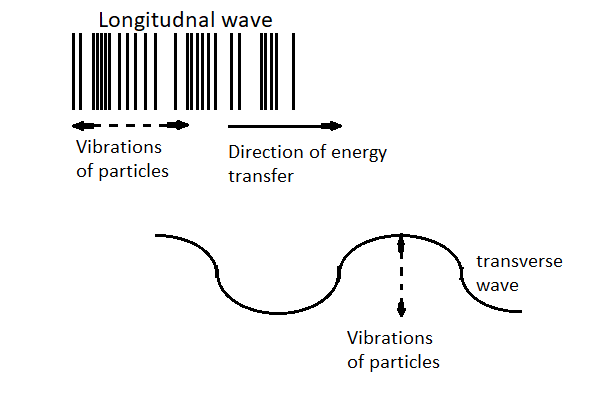
There are three types of waves are present in the physical world which are as follows:
Standing wave
In physics, a standing wave, also known as a stationary wave, is a wave which oscillates in time but whose peak amplitude profile does not move in space. The peak amplitude of the wave oscillations at any point in space is constant with time, and the oscillations at different points throughout the wave are in phase.
For example: sine and cosine wave.
Longitudinal wave
Longitudinal waves are the waves in which the displacement of the medium is in the same direction as, or the opposite direction to, the direction of propagation of the wave, as shown in the figure.
For example: sound waves, waves in the gases.
Transverse wave
In physics, a transverse wave is a moving wave whose oscillations are perpendicular to the direction of the wave or path of propagation as shown in the figure.
For example: Electromagnetic waves.
Now as we know that there are no forces in the gases.
Transverse waves cannot propagate in gases because there are no forces to cause motion perpendicular to the direction of the wave. A transverse wave propagates in a solid because each atom of the solid has an "equilibrium location" where the forces on it from all its neighboring atoms balance.
So wave’s inside the gases are longitudinal.
So this is the required answer.
Hence option (A) is the correct answer.
Note – Gases are composed of matter that has no definite shape and these are free to expand, moreover gases have no fixed volume. Actually the amount of gas that can be filled inside a container is dependent upon the volume of that container. Gases that have no definite shape can be understood by the fact that the same gas can be used to fill a balloon of oval shape and can be used to fill a container shaped cylindrical. This property of gas makes it different from other states of matter.
