Question
Question: Water and turpentine oil (specific heat less than that of water) are both heated to the same tempera...
Water and turpentine oil (specific heat less than that of water) are both heated to the same temperature. Equal amounts of both are then placed in identical calorimeters and then left in air:
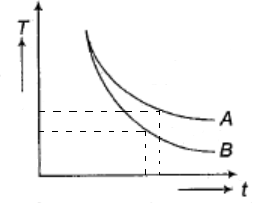
A. A and B will represent cooling curves of water and oil respectively.
B. B and A will represent cooling curves of water and oil respectively.
C. Their cooling curves will be identical.
D. None of the above
Solution
Hint: To correctly identify the cooling curve of water and oil, we use the given information about water and turpentine oil from the question, i.e. the specific heats and temperature from the graph. Using this data we try to find a relation between them at a specific time instance to identify their graphs.
Complete step-by-step solution -
Given Data,
Specific heat of turpentine oil is less than that of the water.
Formula used:
Rate of cooling of a body is given by:
ms.dtdT∝(T - T0)⇒dtdT∝s1, where ‘m’ is the mass of the object, ‘s’ is the specific heat, dtdTis the rate of change of temperature or rate of cooling, T is the temperature of the body, T0is the outside temperature.
Water and Turpentine oil are heated to the same temperature. We know this heat is escaped to the surrounding temperature. The rate at which the heat is lost or the rate of cooling of a body depends on a physical attribute of the substance called specific heat.
Specific heat of a substance is defined as the thermal heat energy required by a substance of unit mass to raise its temperature by one degree Celsius. It is expressed mathematically in form of
c = mΔTΔE, where c is the specific heat, m is the mass of the body, ∆E is the change in energy and ∆T is the change in temperature.
Now from the formula of specific heat and rate of cooling, we can say –
The change in temperature of a body is inversely proportional to its specific heat.
ΔT (rate of cooling) ∝ c (specific heat)1.
Given, the specific heat of oil is less than that of water, i.e. Coil<Cwater
⟹Rate of cooling of oil > Rate of cooling of water.
Therefore, it is clear that oil cools faster than water. So after a time interval after both oil and water began to cool, at a time instant the temperature of water should be more than the temperature of oil.
Looking at the graph, at a time instant the curve with more temperature should be water and less temperature should be oil.
Hence A represents the cooling curve of water and B represents the cooling curve of oil.
Option A is the correct answer.
Note – In order to answer this type of problems the key is to understand the relation between the change in temperature (or the rate of cooling) of a substance to its specific heat capacity. Checking the heat of both the graphs at a specific time instance is a vital step to identify the graph.
The specific heat capacity of water is 4.186 joule gram−1 ∘C. This is generally higher than most of the other substances. This is one of the main reasons water is used as a coolant in various scenarios.
